Abstract
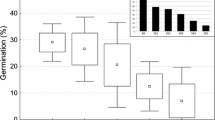
Facilitation of a perennial tussock grass, Ischaemum aristatum var. glaucum, was hypothesized to be the provision of germination safe sites against inundation. We conducted field census and seed addition experiments to test this hypothesis. Facilitation effects of I. aristatum var. glaucum tussocks were obvious; in contrast to 280 seedlings/m2 of ten native species observed on tussocks, seedlings hardly emerged on bare ground, even after seed addition. Although effects of moss occurrence at tussocks were not significant on the total number or species richness of emerged seedlings, significantly positive effects were observed on the seedling survival of some species, including endangered species. Conservation of facilitators will efficiently ensure the regeneration success of native vascular plants.



Similar content being viewed by others
References
Akhalkatsi M, Abdaladze O, Nakhutsrishvili G, Smith WK (2006) Facilitation of seedling microsites by Rhododendron caucaskum extends the Betula litwinowii Alpine treeline, Caucasus Mountains, Republic of Georgia. Arct Antarct Alp Res 38:481–488
Baldwin AH, Egnotovich MS, Clarke E (2001) Hydrologic change and vegetation of tidal freshwater marshes: field, greenhouse, and seed-bank experiments. Wetlands 21:519–531
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielborger K, Travis JMJ, Anthelme F, Armas C, Coll L, Corcket E, Delzon S, Forey E, Kikvidze Z, Olofsson J, Pugnaire F, Quiroz CL, Saccone P, Schiffers K, Seifan M, Touzard B, Michalet R (2008) Facilitation in plant communities: the past, the present, and the future. J Ecol 96:18–34. doi:10.1111/j.1365-2745.2007.01295.x
Bruno JF (2000) Facilitation of cobble beach plant communities through habitat modification by Spartina alterniflora. Ecology 81:1179–1192
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Castellanos EM, Figueroa ME, Davy AJ (1994) Nucleation and facilitation in saltmarsh succession: interactions between Spartina maritima and Arthrocnemum perenne. J Ecol 82:239–248
Castro J, Zamora R, Hodar JA, Gomez JM (2002) Use of shrubs as nurse plants: a new technique for reforestation in Mediterranean mountains. Restor Ecol 10:297–305
Crain CM, Bertness ND (2005) Community impacts of a tussock sedge: is ecosystem engineering important in benign habitats? Ecology 86:2695–2704
Ervin GN (2007) An experimental study on the facilitative effects of tussock structure among wetland plants. Wetlands 27:620–630
Fogel BN, Crain CM, Bertness MD (2004) Community level engineering effects of Triglochin maritima (seaside arrowgrass) in a salt marsh in northern New England, USA. J Ecol 92:589–597
Griffith AB, Forseth IN (2003) Establishment and reproduction of Aeschynomene virginica (L.) (Fabaceae) a rare, annual, wetland species in relation to vegetation removal and water level. Plant Ecol 167:117–125
Groeneveld EVG, Masse A, Rochefort L (2007) Polytrichum strictum as a nurse-plant in peatland restoration. Restor Ecol 15:709–719
Halpern BS, Silliman BR, Olden JD, Bruno JP, Bertness MD (2007) Incorporating positive interactions in aquatic restoration and conservation. Front Ecol Environ 5:153–160
Hopfensperger KN, Engelhardt KAM (2007) Coexistence of Typha angustifolia and Impatiens capensis in a tidal freshwater marsh. Wetlands 27:561–569
Neff KP, Rusello K, Baldwin AH (2009) Rapid seed bank development in restored tidal freshwater wetlands. Restor Ecol 17:539–548. doi:10.1111/j.1526-100X.2008.00415.x
Nishihiro J, Washitani I (2009) Quantitative evaluation of water-level effects on “regeneration safe-sites” for lakeshore plants in Lake Kasumigaura, Japan. Lake Reserv Manag 25:217–223. doi:10.1080/07438140902938332
Nishihiro J, Araki S, Fujiwara N, Washitani I (2004a) Germination characteristics of lakeshore plants under an artificially stabilized water regime. Aquat Bot 79:333–343. doi:10.1016/j.aquabot.2004.05.005
Nishihiro J, Miyawaki S, Fujiwara N, Washitani I (2004b) Regeneration failure of lakeshore plants under an artificially altered water regime. Ecol Res 19:613–623
Nozoe K, Nishihiro J, Hotes S, Washitani I (2010) Importance of Ischaemum aristatum var. glaucum as an indicator of plant species richness in Myoginohana Marsh, Lake Kasumigaura, Japan (in Japanese with English summary). Jpn J Conserv Ecol 15:281–290
Padilla FM, Pugnaire FI (2006) The role of nurse plants in the restoration of degraded environments. Front Ecol Environ 4:196–202
Peach M, Zedler JB (2006) How tussocks structure sedge meadow vegetation. Wetlands 26:322–335
Peterson JE, Baldwin AH (2004) Seedling emergence from seed banks of tidal freshwater wetlands: response to inundation and sedimentation. Aquat Bot 78:243–254. doi:10.1016/j.aquabot.2003.10.005
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Scarano FR, Ribeiro KT, De Moraes LFD, De Lima HC (1997) Plant establishment on flooded and unflooded patches of a freshwater swamp forest in southeastern Brazil. J Trop Ecol 13:793–803
van de Koppel J, Crain CM (2006) Scale-dependent inhibition drives regular tussock spacing in a freshwater marsh. Am Nat 168:E136–E147
van Tooren BF (1988) The fate of seeds after dispersal in chalk grassland: the role of the bryophyte layer. Oikos 53:41–48
Wang Z, Nishihiro J, Washitani I (2011) Facilitation of plant species richness and endangered species by a tussock grass in a moist tall grassland revealed using hierarchical Bayesian analysis. Ecol Res. doi:10.1007/s11284-011-0862-z
Yabe K (1985) Distribution and formation of tussocks in Mobara-Yatsumi Marsh. Jpn J Ecol 35:183–192
Acknowledgments
We thank Ms. Mihoko Uzawa of Ibaraki Nature Museum, Japan for providing information on mosses. This research was financed partly by a Grant-in-Aid for Young Scientists (B) 22710232 and Environment Research and Technology Development Fund (S9) of the Ministry of the Environment, Japan to J.N.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, Z., Nishihiro, J. & Washitani, I. Regeneration of native vascular plants facilitated by Ischaemum aristatum var. glaucum tussocks: an experimental demonstration. Ecol Res 27, 239–244 (2012). https://doi.org/10.1007/s11284-011-0897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-011-0897-1