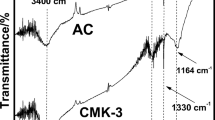
The effect of the conditions of postsynthetic modification of CMK-3 carbon mesoporous molecular sieves on their structural and adsorption properties was studied. The specific surface, volume, pore size, and hydrogen adsorption are markedly enhanced upon activation of CMK-3 by thermal, steam, and chemical treatment using H2, CO2, H2O2, and HNO3. Analysis of the occupancy density of the mesopore surface indicated increased hydrogen adsorption capacity of the hydrogen-activated carbon surface of CMK-3. Hydrogen adsorption is increased from 1.20 to 2.23 mass % at 1 atm and 77 K by steam treatment. This effect may be employed to create efficient carbon MMS adsorbents, including composite adsorbents, for the accumulation and storage of hydrogen at high pressure (adsorption >6 mass %).
Similar content being viewed by others
References
S. H. Joo, S. Jun, and R. Ryoo, Micropor. Mesopor. Mater., 44-45, 153-158 (2001).
R. Ryoo, S. H. Joo, M. Kruk, et al., Adv. Mater., 13, No. 9, 677-681 (2001).
H. C. Foley, J. Micropor. Mater., 4, 407-415 (1995).
K. Xia, Q. Gao, Ch. Wu, et al., Carbon, 45, 1989-1996 (2007).
R. Gadiou, S. Saadallah, T. Piquerot, et al., Micropor. Mesopor. Mater., 79, 121-127 (2005).
M. Kruk, K. M. Kohlhaas, B. Dufour, et al., Micropor. Mesopor. Mater., 102, 178-187 (2007).
N. D. Lysenko, M. V. Opanasenko, P. S. Yaremov, et al., Teor. Éksp. Khim., (2009), in print.
S. G. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, Academic Press, New York (1982), p. 94.
D. Dollimore and G. R. Heal, J. Appl. Chem., 14, 109 (1964).
G. Horvath and K. Kawazoe, J. Chem. Eng. Jpn., 16, No. 6, 470-475 (1983).
D. Zhao, J. Feng, Q. Huo, et al., Science, 279, 548-560 (1998).
A. Pacula and R. Mokaya, J. Phys. Chem., C, 112, 2764-2769 (2008).
M. M. Dubinin and H. F. Stoeckli, J. Colloid Interface Sci., 75, 1-10 (1980).
P. S. Yaremov and V. G. Il’in, Teor. Éksp. Khim., 44, No. 2, 67-73 (2008). [Theor. Experim. Chem., 44, No. 2, 67-74 (2008).]
Z. Yang, Y. Xia, and R. Mokaya, J. Am. Chem. Soc., 129, No. 6, 1673-1676 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 45, No. 6, pp. 365-370, November-December, 2009.
Rights and permissions
About this article
Cite this article
Lysenko, N.D., Yaremov, P.S., Shvets, A.V. et al. Effect of the chemical and structural modification of CMK-3 mesoporous carbon molecular sieve on hydrogen adsorption. Theor Exp Chem 45, 380–385 (2009). https://doi.org/10.1007/s11237-010-9110-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-010-9110-9