Abstract
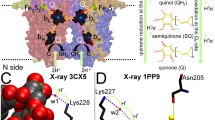
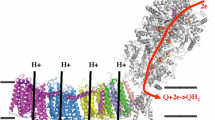
At the heart of the Q cycle hypothesis, the cytochrome bc 1 complex (bc 1) is required to separate the two electrons from a quinol molecule at the quinol oxidation site. Recent studies have brought to light an intricate mechanism for this bifurcated electron transfer. A survey of the protein data bank shows 30 entries for the structures of bc 1 and the homologous b 6 f complex. These structures provide considerable insights into the structural organization of mitochondrial, bacterial, and plant enzymes. Crystallographic binding studies of bc 1 with either quinone reduction (QN) and/or quinol oxidation (QP) site inhibitors offer atomic details on how these compounds interact with residues at their respective sites. Most importantly, the different locations and apparent flexibility observed in crystals for the extrinsic domain of the iron-sulfur protein (ISP) subunit suggest a mechanism for electron bifurcation at the QP site. Analyses of various inhibitor-bound structures revealed two classes of QP site inhibitors: Pm inhibitors that promote ISP mobility and Pf inhibitors that favor the fixation of the ISP conformation. Those analyses also shed light on a possible process by which the ISP motion switch is controlled. The first phase reduction of ISP is shown to be comparable to the reduction of the b L heme by pre-steady state kinetic analysis, whereas the second phase reduction of ISP share similar kinetics with the reduction of the b H heme. The reduction of cyt c 1 is measured much slower, indicating that the reduced ISP remains bound at the QP site until the reduced heme b L is oxidized by the heme b H and supporting the existence of a control mechanism for the ISP motion switch.









Similar content being viewed by others
Abbreviations
- 2Fe2S :
-
Two-iron-two-sulfur cluster of ISP
- bc 1 :
-
Ubiquinol cytochrome c oxidoreductase
- b H :
-
High potential b heme
- b L :
-
Low potential b heme
- cyt b :
-
Cytochrome b subunit
- cyt c 1 :
-
Cytochrome c 1 subunit
- EPR:
-
Electron paramagnetic resonance
- ET:
-
Electron transfer
- IMS:
-
Intermembrane space
- ISP:
-
Iron-sulfur protein subunit
- ISP-ED:
-
The extrinsic domain of ISP
- MOA:
-
β-Methoxyacrylate
- NQNO:
-
2-Nonyl-4-hydroxyquinoline N-oxide
- Pf:
-
Quinol oxidation site inhibitors that fix ISP-ED conformation
- Pm:
-
Quinol oxidation site inhibitors that promote ISP-ED movement
- QP :
-
Quinol oxidation
- QN :
-
Quinone reduction
- QH2 :
-
Ubiquinol
- rms deviation:
-
Root mean square deviation
- Rsbc 1 :
-
Rhodobacter sphaeroides bc 1
- TM:
-
Transmembrane
- UHDBT:
-
5-Undecyl-6-hydroxy-4,7-dioxobenzothiazol
References
Akiba T, Toyoshima C, Matsunaga T, Kawamoto M, Kubota T, Fukuyama K, Namba K, Matsubara H (1996) Three-dimensional structure of bovine cytochrome bc 1 complex by electron cryomicroscopy and helical image reconstruction. Nat Struct Biol 3:553–561
Atta-Asafo-Adjei E, Daldal F (1991) Size of the amino acid side chain at position 158 of cytochrome b is critical for an active cytochrome bc 1 complex and for photosynthetic growth of Rhodobacter capsulatus. Proc Natl Acad Sci USA 88:492–496
Berry EA, Huang L, Earnest T, Jap BK (1992) X-ray diffraction by crystals of beef heart ubiquinol: cytochrome c oxidoreductase. J Mol Biol 224:1161–1166
Berry EA, Shulmeister VM, Huang LS, Kim SH (1995) A new crytal form of bovine heart ubiquinol:cytochrome c oxidoreductase: determination of space group and unit-cell parameters. Acta Cryst D51:235–239
Berry EA, Huang L, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F (2004) X-ray structure of Rhodobacter capsulatus cytochrome bc 1: comparison with its mitochondrial and chloroplast counterparts. Photosyn Res 81:251–275
Bowyer JR, Dutton PL, Prince RC, Crofts AR (1980) The role of the Rieske iron–sulfur center as the electron donor to ferricytochrome c2 in Rhodopseudomonas sphaeroides. Biochim Biophys Acta 592:445–460
Brandt U (1998) The chemistry and mechanics of ubihydroquinone oxidation at center P (Qo) of the cytochrome bc 1 complex. Biochim Biophys Acta 1365:261–268
Brandt U, von Jagow G (1991) Analysis of inhibitor binding to the mitochondrial cytochrome c reductase by fluorescence quench titration. Eur J Biochem 195:163–170
Brandt U, Okun JG (1997) Role of deprotonation events in ubihydroquinone:cytochrome c oxidoreductase from bovine heart and yeast mitochondria. Biochemistry 36:11234–11240
Brandt U, Haase U, Schagger H, von Jagow G (1991) Significance of the “Rieske” iron–sulfur protein for formation and function of the ubiquinol-oxidation pocket of mitochondrial cytochrome c reductase (bc 1 complex). J Biol Chem 266:19958–19964
Bruel C, di Rago J, Slonimski PP, Lemesle-Meunier D (1995) Role of the evolutionarily conserved cytochrome b tryptophan 142 in the ubiquinol oxidation catalyzed by the bc 1 complex in the yeast Saccharomyces cerevisiae. J Biol Chem 270:22321–22328
Crofts AR, Barquera B, Bechmann G, Guergova M, Salcedo-Hernandez R, Hacker B, Hong S, Gennis RB, Mathis P (1995) Photosynthesis: from light to biosphere. Kluwer Academic Publications, The Netherlands
Crofts AR, Hong S, Zhang Z, Berry EA (1999) Physicochemical aspects of the movement of the Rieske iron sulfur protein during quinol oxidation by teh bc 1 complex from mitochondria and photosynthetic bacteria. Biochemistry 38:15827–15839
Crofts AR, Shinkarev VP, Kolling DR, Hong S (2003) The modified Q-cycle explains the apparent mismatch between the kinetics of reduction of cytochromes c1 and bH in the bc 1 complex. J Biol Chem 278:36191–36201
Darrouzet E, Valkova-Valchanova M, Daldal F (2000a) Probing the role of the Fe–S subunit hinge region during Q(o) site catalysis in Rhodobacter capsulatus bc(1) complex. Biochemistry 39:15475–15483
Darrouzet E, Valkova-Valchanova M, Moser CC, Dutton PL, Daldal F (2000b) Uncovering the [2Fe2S] domain movement in cytochrome bc 1 and its implications for energy conversion. Proc Natl Acad Sci USA 97:4567–4572
De Vries S, Albracht SPJ, Berden JA, Slater EC (1981) A new species of bound ubisemiquinone anion in QH2: cytochrome c oxidoreductase. J Biol Chem 256:11996–11998
Ding H, Robertson DE, Daldal F, Dutton PL (1992) Cytochrome bc 1 complex [2Fe-2S] cluster and its interaction with ubiquinone and ubihydroquinone at the Qo site: a double-occupancy Qo site model. Biochemistry 31:3144–3158
Esser L, Quinn B, Li Y, Zhang M, Elberry M, Yu L, Yu CA, Xia D (2004) Crystallographic studies of quinol oxidation site inhibitors: a modified classification of inhibitors for the cytochrome bc 1 complex. J Mol Biol 341:281–302
Esser L, Gong X, Yang S, Yu L, Yu CA, Xia D (2006) Surface-modulated motion switch: capture and release of iron-sulfur protein in the cytochrome bc 1 complex. Proc Natl Acad Sci USA 103:13045–13050
Farid RS, Moser CC, Dutton PL (1993) Electron transfer in proteins. Curr Opin Struct Biol 3:225–233
Gao X, Wen X, Yu C, Esser L, Tsao S, Quinn B, Zhang L, Yu L, Xia D (2002) The crystal structure of mitochondrial cytochrome bc 1 in complex with famoxaodne: the role of aromatic–aromatic interaction in inhibition. Biochemistry 41:11692–11702
Gao X, Wen X, Esser L, Yu L, Yu CA, Xia D (2003) Structural basis for the quinone reduction in bc 1 complex: a comparative analysis of crystal structures of mitochondrial cytochrome bc 1 with bound substrate and inhibitors. Biochemistry 42:9067–9080
Geier BM, Haase U, von Jagow G (1993) Inhibitor binding to the Qp-site of bc 1 complex: comparative studies of yeast mutants and natural inhibitor resistant fungi. Biochem Soc Trans 22:203–209
Gurung B, Yu L, Xia D, Yu CA (2005) The iron-sulfur cluster of the Rieske iron-sulfur protein functions as a proton-exiting gate in teh cytochrome bc(1) complex. J Biol Chem 280:24895–24902
Huang L, Cobessi D, Tung EY, Berry EA (2005) Binding of the respiratory chain inhibitor antimycin to the mitochondrial bc 1 complex: a new crystal structure reveals an altered intramolecular hydrogen-bonding pattern. J Mol Biol 351:573–597
Hunte C, Koepke J, Lange C, Rossmanith T, Michel H (2000) Structure at 2.3 A resolution of the cytochrome bc(1) complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure 15:669–684
Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc 1 complex [see comments]. Science 281:64–71
von Jagow G, Link TA (1986) Use of specific inhibitors on the mitochondrial bc 1 complex. Meth Enzymol 126:253–271
von Jagow G, Ohnishi T (1985) The chromone inhibitor stigmatellin - binding to the ubiquinol oxidation center at the C-side of the mitochondrial membrane. FEBS Lett 185:311–315
Junemann S, Heathcote P, Rich PR (1998) On the mechanism of quinol oxidation in the bc 1 complex. J Biol Chem 273:21603–21607
Kim H, Xia D, Yu CA, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J (1998) Inhibitor binding changes domain mobility in the iron-sulfur protein of the mitochondrial bc 1 complex from bovine heart. Proc Natl Acad Sci USA 95:8026–8033
Kurisu G, Zhang H, Smith JL, Cramer WA (2003) Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302:1009–1014
Lange C, Nett JH, Trumpower BL, Hunte C (2001) Specific roles of protein-phospholipid interactions in the yeast cytochrome bc 1 complex structure. EMBO J 20:6591–6600
Lee JW, Chan M, Law TV, Kwon HJ, Jap BK (1995) Preliminary cryocrystallographic study of the mitochondrial cytochrome bc 1 complex:improved crystallization and flash-colling of a large membrane protein. J Mol Biol 252:15–19
Lemesle-Meunier D, Brivet-Chevillotte P, di Rago JP, Slonimski PP, Bruel C, Tron T, Forget N (1993) Cytochrome b-deficient mutants of the ubiquinol-cytochrome c oxidoreductase in Saccharomyces cerevisiae. Consequence for the functional and structural characteristics of the complex. J Biol Chem 268:15626–15632
Leonard K, Wingfield P, Arad T, Weiss H (1981) Three-dimensional structure of ubiquinol:cytochrome c reductase from neurospora mitochondria determined by electron microscopy of membrane crystals. J Mol Biol 149:259–274
Link TA (1997) The role of the ‘Rieske’ iron sulfur protein in the hydroquinone oxidation (Qp) site of the cytochrome bc 1 complex - the ‘proton-gated affinity change’ machanism. FEBS Lett 412:257–264
Link TA, Haase U, Brandt U, von Jagow G (1993) What information do inhibitors provide about the structure of the hydroquinone oxidation site of ubihydroquinone: cytochrome c oxidoreductase? J Bioenerg Biomembr 25:221–232
Mather MW, Yu L, Yu CA (1995) The involvement of threonine 160 of cytochrome b of Rhodobacter sphaeroides cytochrome bc 1 complex in quinone binding and interaction with subunit IV. J Biol Chem 270:28668–28675
Mitchell P (1976) Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol 62:327–367
Nett JH, Hunte C, Trumpower BL (2000) Changes to the length of the flexible linker region of the Rieske protein impair the interaction of ubiquinol with the cytochrome bc 1 complex. Eur J Biochem 267:5777–5782
Obungu VH, Wang Y, Amyot SM, Gocke CB, Beattie DS (2000) Mutations in the tether region of the iron-sulfur protein affect the activity and assembly of the cytochrome bc(1) complex of yeast mitochondria. Biochim Biophys Acta 1457:36–44
Palsdottir H, Lojero CG, Trumpower BL, Hunte C (2003) Structure of the yeast cytochrome bc 1 complex with a hydroxyquinone anion Qo site inhibitor bound. J Biol Chem 278:31303–31311
Sadoski RC, Engstrom G, Tian H, Zhang L, Yu CA, Yu L, Durham B, Millett F (2000) Photoinduced electron-transfer between the rieske iron-sulfur protein and cytochrome c1 in the cytochrome bc 1 complex. Biochemistry 39:4231–4236
Stroebel D, Choquet Y, Popot JL, Picot D (2003) An atypical haem in the cytochrome b(6)f complex. Nature 426:413–418
Tian H, Yu L, Mather MW, Yu CA (1997) The involvement of serine 175 and alanine 185 of cytochrome b of Rhodobacter sphaeroides cytochrome bc 1 complex in interaction with iron-sulfur protein. J Biol Chem 272:23722–23728
Tian H, Yu L, Mather MW, Yu CA (1998) Flexibility of the neck region of the rieske iron-sulfur protein is functionally important in the cytochrome bc 1 complex. J Biol Chem 273:27953–27959
Tian H, White S, Yu L, Yu CA (1999) Evidence for the head domain movement of the Rieske iron-sulfur protein in electron transfer reaction of the cytochrome bc 1 complex. J Biol Chem 274:7146–7152
Trumpower BL (1990) The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc 1 complex. J Biol Chem 265:11409–11412
Trumpower BL (2002) A concerted, alternating sites mechanism of ubiquinol oxidation by the dimeric cytochrome bc 1 complex. Biochim Biophys Acta 1555:166–173
Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J (1997) Crystal structure of the cytochrome bc 1 complex from bovine heart mitochondria. Science 277:60–66
Xiao K, Yu L, Yu CA (2000) Confirmation of the involvement of protein domain movement during the catalytic cycle of the cytochrome bc 1 complex by the formation of the intersubunit disulfide bond between cytochrome b and the iron-sulfur protein. J Biol Chem 275:38597–38604
Yan J, Cramer WA (2003) Functional insensitivity of the cytochrome b6f complex to structure changes in the hinge region of the Rieske iron-sulfur protein. J Biol Chem 278:20925–20933
Yu CA, Yu L (1993) Mitochondrial ubiquinol-cytochrome c reductase complex: crystallization and protein: ubiquinone interaction. J Bioenerg Biomembr 25:259–273
Yu CA, Xia JZ, Kachurin AM, Yu L, Xia D, Kim H, Deisenhofer J (1996) Crystallization and preliminary structure of beef heart mitochondrial cytochrome-bc 1 complex. Biochim Biophys Acta 1275:47–53
Yu CA, Wen X, Xiao K, Xia D, Yu L (2002) Inter- and intra-molecular electron transfer in the cytochrome bc 1 complex. Biochim Biophys Acta 1555:65–70
Yue WH, Zou YP, Yu L, Yu CA (1991) Crystallization of mitochondrial ubiquinol-cytochrome c reductase. Biochemistry 30:2303–2306
Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA, Kim SH (1998) Electron transfer by domain movement in cytochrome bc 1. Nature 392:677–684
Zhu J, Egawa T, Yeh S, Yu L, Yu CA (2007) Simultaneous reduction of iron-sulfur protein and cytochrome b L during ubiquinol oxidation in cytochrome bc 1 complex. PNAS 104:4864–4869
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and in part from a NIH grant (GM 30721) to CAY. The authors wish to thank George Leiman for editorial assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xia, D., Esser, L., Yu, L. et al. Structural basis for the mechanism of electron bifurcation at the quinol oxidation site of the cytochrome bc 1 complex. Photosynth Res 92, 17–34 (2007). https://doi.org/10.1007/s11120-007-9155-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-007-9155-3