Abstract
A computer model comprising light reactions, electron–proton transport, enzymatic reactions, and regulatory functions of C3 photosynthesis has been developed as a system of differential budget equations for intermediate compounds. The emphasis is on electron transport through PSII and PSI and on the modeling of Chl fluorescence and 810 nm absorptance signals. Non-photochemical quenching of PSII excitation is controlled by lumenal pH. Alternative electron transport is modeled as the Mehler type O2 reduction plus the malate-oxaloacetate shuttle based on the chloroplast malate dehydrogenase. Carbon reduction enzymes are redox-controlled by the ferredoxin–thioredoxin system, sucrose synthesis is controlled by the fructose 2,6-bisphosphate inhibition of cytosolic FBPase, and starch synthesis is controlled by ADP-glucose pyrophosphorylase. Photorespiratory glycolate pathway is included in an integrated way, sufficient to reproduce steady-state rates of photorespiration. Rate-equations are designed on principles of multisubstrate-multiproduct enzyme kinetics. The parameters of the model were adopted from literature or were estimated from fitting the photosynthetic rate and pool sizes to experimental data. The model provided good simulations for steady-state photosynthesis, Chl fluorescence, and 810 nm transmittance signals under varying light, CO2 and O2 concentrations, as well as for the transients of post-illumination CO2 uptake, Chl fluorescence induction and the 810 nm signal. The modeling shows that the present understanding of photosynthesis incorporated in the model is basically correct, but still insufficient to reproduce the dark-light induction of photosynthesis, the time kinetics of non-photochemical quenching, ‘photosynthetic control’ of plastoquinone oxidation, cyclic electron flow around PSI, oscillations in photosynthesis. The model may find application for predicting the results of gene transformations, the analysis of kinetic experimental data, the training of students.









Similar content being viewed by others
References
Badger MR, Lorimer GH (1981) Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry 20(8):2219–2225
Bassham JA, Jensen RG (1967) Photosynthesis of carbon compounds. In: San Pietro A, Greer FA, Army TJ (eds) Harvesting the Sun. Academic Press, New York/London, pp 79–110
Brown HT, Escombe ELS (1900) Static diffusion of gases and liquids in relation to the assimilation of carbon and translocation in plants. Phil Trans 193:223–291
Buckley TN, Farquhar GD (2004) A new analytical model for whole-leaf electron transport rate. Plant Cell Environ 27:1487–1502
Bukhov N, Egorova E, Carpentier R (2002) Electron flow to photosystem I from stromal reductantsin vivo: the size of the pool of stromal reductants controls the rate of electron donation to both rapidly and slowly reducing photosystem I units. Planta 215:812–820
von Caemmerer S, Furbank RT (1999) Modelling C4 photosynthesis. In: Sage RF, Monson RK (eds) C4 plant biology. Academic Press, San Diego/London/Boston/NewYork/Sydney/Tokyo/Toronto, pp 173–211
von Caemmerer S (2000) Biochemical models of leaf photosynthesis. Australia, CSIRO Publishing
Chartier P (1966) Etude theorique de l’assimilation brute de la feuille. Ann Physiol Veg 8:167–195
Cleland WW (1963) The kinetic of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta 767:432–443
Cramer WA, Soriano GM, Ponomarev M, Huang D, Zhang H, Martinez SE, Smith JL (1996) Some new structural aspects and old controversies concenrning the cytochrome b 6 f complex of oxygenic photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 47:477–508
Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM (2001) Contribution of electric field (Δψ) to steady-state transthylakoid proton motive force (pmf) in vitro and in vivo. Control of pmf parsing into Δ ψ and ΔpH by ionic strength. Biochemistry 40:1226–1237
Eichelmann H, Laisk A (1999) Ribulose-1,5-bisphosphate carboxylase/oxygenase content, assimilatory charge and mesophyll conductance in leaves. Plant Physiol 119:179–189
Eichelmann H, Laisk A (2000) Cooperation of photosystems II and I in leaves as analysed by simultaneous measurements of chlorophyll fluorescence and transmittance at 800 nm. Plant Cell Physiol 41:138–147
Eichelmann H, Weis E, Laisk A (1990) The effect of electron cycling around PSII on fluorescence induction mathematical modelling. In: Baltscheffsky M (ed) Current research in photosynthesis, vol I. Kluwer Academic Publishers, Dordrecht, the Netherlands, pp 663–666
Farquhar GD (1979) Models describing the kinetics of ribulose bisphosphate carboxylase-oxygenase. Arch Biochem Biophys 2:456–468
Farquhar GD, von Caemmerer S (1982) Modelling of photosynthetic response to environmental conditions. In Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology. Encycl Plant Physiol, New Series, vol. 12B, pp 549–588. Springer-Verlag, Berlin
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Feniouk BA, Mulkidjanian AY, Junge W (2005) Proton slip in the ATP synthase of Rhodobacter capsulatus: induction, proton conduction, and nucleotide dependence. Biochim Biophys Acta 1706:184–194
Flügge U-I (1991) Metabolite translocators of the chloroplast envelope. Annu Rev Plant Physiol Plant Mol Biol 42:129–144
Fridlyand LE, Backhausen JE, Scheibe R (1999) Homeostatic regulation upon changes of enzyme activities in the Calvin cycle as an example for general mechanisms of flux control. What can we expect from transgenic plants? Photosynth Res 61(3):227–239
Hahn BD (1987) A matemathical model of photorespiration and photosynthesis. Ann Bot 60:157–169
Harbinson J, Hedley CL (1989) The kinetics of P700+ reduction in leaves: a novel in situ probe of thylakoid functioning. Plant Cell Environ 12:357–369
Heber U, Neimanis S, Dietz KJ, Viil J (1986) Assimilatory power as a driving force in photosynthesis. Biochem Biophys Acta 852: 144–155
Heimann S, Klughammer C, Schreiber U (1998) Two distinct states of the thylakoid bf complex. FEBS Lett 426:126–130
Holzhütter H-G (2004) The principle of flux minimization and its application to estimate stationary fluxes in metabolic networks. Eur J Biochem 271:2905–2922
Horton P, Ruban AV (1992) Regulation of photosystem II. Photosynth Res 34:375–385
Johnson G (2003) Thiol regulation of the thylakoid electron transport chain – a missing link in the regulation of photosynthesis. Biochemistry 42:3040–3044
Jordan DB, Chollet R, Ogren WL (1983) Binding of phosphorylated effectors by active and inactive forms of ribulose-1,5-bisphosphate carboxylase. Biochemistry 22:3410–3418
Kanazawa A, Kramer DM (2002) In vivomodulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. PNAS 99:12789–12794
Kirchhoff H, Schöttler MA, Maurer J, Weis E (2004) Plastocyanin redox kinetics in spinach chloroplasts: evidence for disequilibrium in the high potential chain. Biochim Biophys Acta 1659:63–72
Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192:261–268
Krause GH, Heber U (1976) Energetics of intact chloroplasts. In: Barber J (ed) The intact chloroplast. Elsevier/North-Holland Biomedical Press, Amsterdam/New York/Oxford, pp 171–214
Kull O, Kruijt B (1998) Leaf photosynthetic light response: a mechanistic model for scaling photosynthesis to leaves and canopies. Funct Ecol 12:767–777
Kull O, Kruijt B (1999) Acclimation of photosynthesis to light: a mechanistic approach. Functional Ecol 13(1): 24
Laisk A (1970) A model of leaf photosynthesis and photorespiration. In: Shetlik I (ed) Prediction and measurement of photosynthetic productivity. PUDOC, Wageningen, pp 295–306
Laisk A (1977) Modelling of the closed Calvin cycle. In: Unger K (ed) Biophysikalische Analyse Pflanzlicher Systeme. VEB Fischer-Verlag, Jena, DDR, pp 175–182
Laisk A, Edwards GE (2000) A mathematical model of C4 photosynthesis: the mechanism of concentrating CO2 in NADP-malic enzyme type species. Photosynth Res 66:199–224
Laisk A, Eichelmann H (1989) Towards understanding oscillations: a mathematical model of the biochemistry of photosynthesis. Phil Trans R Soc Lond 323:369–384
Laisk A, Eichelmann H, Oja V, Eatherall A, Walker DA (1989) A mathematical model of the carbon metabolism in photosynthesis. Difficulties in explaining oscillations by fructose 2,6-bisphosphatase. Proc R Soc Lond B 237:389–415
Laisk A, Eichelmann H, Oja V, Peterson RB (2005) Control of cytochrome b 6 f at low and high light intensity and cyclic electron transport in leaves. Biochim Biophys Acta 1708:79–90
Laisk A, Eichelmann H, Oja V, Rasulov B, Rämma H (2006) Photosystem II cycle and alternative electron flow in leaves. Plant Cell Physiol 47, in press
Laisk A, Kiirats O, Eichelmann H, Oja V (1987) Gas exchange studies of carboxylation kinetics in intact leaves. In: Biggins J (ed) Progress in photosynthesis research. Martinus Nijhoff Publishers, Dordrecht, the Netherlands, pp 245–252
Laisk A, Laarin P (1983) Feedback regulation of the potential rate of photosynthesis. In: Margna U (ed) Regulation of Plant Growth and Metabolism. Valgus Publishing, Tallinn, pp 135–150 (in Russian)
Laisk A, Oja V (1994) Range of the photosynthetic control of postillumination P700 reduction rate in sunflower leaves. Photosynth Res 39:39–50
Laisk A, Oja V (1995) Coregulation of electron transport through PS I by Cyt b 6 f, excitation capture by P700 and acceptor side reduction. Time kinetics and electron transport requirement. Photosynth Res 45:11–19
Laisk A, Oja V (1998) Dynamic gas exchange of leaf photosynthesis. Measurement and interpretation. CSIRO Publishing, Canberra
Laisk A, Oja V (2000a) Alteration of PSII properties with non-photochemical excitation quenching. Phil Trans R Soc Lond B 355:1405–1418
Laisk A, Oja V (2000b) Electron transport through photosystem II in leaves during light pulses: acceptor resistance increases with nonphotochemical excitation quenching. Biochim Biophys Acta 1460:255–267
Laisk A, Oja V, Kiirats O (1984) Assimilatory power (post-illumination CO2 uptake) in leaves—measurement, environmental dependencies and kinetic properties. Plant Physiol 76:723–729
Laisk A, Oja V, Rasulov B, Eichelmann H, Sumberg A (1997) Quantum yields and rate constants of photochemical and nonphotochemical excitation quenching. Experiment and model. Plant Physiol 115:803–815
Laisk A, Oja V, Rasulov B, Rämma H, Eichelmann H, Kasparova I, Pettai H, Padu E, Vapaavuori E (2002) A computer-operated routine of gas exchange and optical measurements to diagnose photosynthetic apparatus in leaves. Plant Cell Environ 25:923–943
Laisk A, Walker DA (1986) Control of phosphate turnover as a rate-limiting factor and possible cause of oscillations in photosynthesis: a mathematical model. Proc R Soc Lond B 227:281–302
Laisk A, Walker DA (1989) A mathematical model of electron transport. Thermodynamic necessity for photosystem II regulation: "light stomata". Proc R Soc Lond B 237:417–444
Makino A, Mae T, Ohira K (1987) Variation in the contents and kinetic properties of ribulose-1,5-bisphosphate carboxylases among rice species. Plant Cell Physiol 28:799–804
Makino A, Miyake C, Yokota A (2002) Physiological functions of the water-water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. Plant Cell Physiol 43:1017–1026
Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev 41:445–502
Miyake C, Yokota A (2000) Determination of the rate of photoreduction of O2 in the water-water cycle in watermelon leaves and enhancement of the rate by limitation of photosynthesis. Plant Cell Physiol 41(3):335–343
Mott KA, Woodrow IE (2000) Modelling the role of Rubisco activase in limiting non-steady-state photosynthesis. J Exp Bot 51:399–406
Niyogi KK, Li X-P, Rosenberg V, Jung H-S (2005) Is PsbS the site of non-photochemical quenching in photosynthesis? J Exp Bot 56:375–382
Oja V, Bichele I, Hüve K, Rasulov B, Laisk A (2004) Reductive titration of photosystem I and differential extinction coefficient of P700+ at 810–950 nm in leaves. Biochim Biophys Acta 1658:225–234
Oja V, Eichelmann H, Peterson RB, Rasulov B, Laisk A (2003) Decyphering the 820 nm signal: redox state of donor side and quantum yield of photosystem I in leaves. Photosynth Res 78:1–15
Oja V, Laisk A (1995) Gas system and method for CO2 titration of intact leaves. Photosynthetica 31:37–50
Oja V, Laisk A (2000) Oxygen yield from single turnover flashes in leaves:non-photochemical excitation quenching and the number of active PSII. Biochim Biophys Acta 1460:291–301
Oja V, Laisk A, Heber U (1986) Light-induced alkalization of the chloroplast stroma in vivo as estimated from the CO2 capacity of intact sunflower leaves. Biochim Biophys Acta 849:355–365
Pärnik T, Keerberg O (1995) Decarboxylation of primary and end products of photosynthesis at different oxygen concentrations. J Exp Bot 46:1439–1447
Peltier G, Cournac L (2002) Chlororespiration. Annu Rev Plant Biol 53:523–550
Pettai H, Oja V, Freiberg A, Laisk. A (2005) Photosynthetic activity of far-red light in green plants. Biochim Biophys Acta 1708:311–321
Pettersson G, Ryde-Pettersson U (1988) A mathematical model of the Calvin photosynthesis cycle. Eur J Biochem 175:661–672
Porcar-Castell A, Bäck J, Juurola E, Hari P (2006) Dynamics of the energy flow through photosystem II under changing light conditions: a model approach. Functional Plant Biol 33:229–239
Rabinowitch E (1953) Photosynthesis II. Publ. House of Foreign Liter., Moscow.
Rees D, Noctor G, Ruban AV, Crofts J, Young A, Horton P (1992) pH dependent chlorophyll fluorescence quenching in spinach thylakoids from light treated or dark adapted leaves. Photosynth Res 31:11–19
Rumberg B, Schubert K, Strelow F, Tran-Anh T (1990) The H+/ATP coupling ratio at the H+-ATP-synthase of spinach chloroplasts is four. In: Baltscheffsky M (ed) Current research in photosynthesis, vol III. Kluwer Acad. Publ., the Netherlands, pp 125–128
Sacksteder CA, Kanazawa A, Jacoby ME, Kramer DM (2000) The protonto electron stoichiometry of steady-state photosynthesis in living plants: a proton-pumping Q cycle is continuously engaged. PNAS 97:14283–14288
Scheibe R (1987) NADP+-malate dehydrogenase in C3-plants: Regulation and role of a light-activated enzyme. Physiol. Plantarum 71:393–400
Scheuring S, Fotiadis D, Möller C, Müller SA, Engel A, Müller DJ (2001) Single proteins observed by atomic force microscopy. Single Mol 2:59–67
Seelert H, Poetsch A, Dencher NA, Engel A, Stahlberg H, Müller DJ (2000) Proton powered turbine of a plant motor. Nature 405:418–419
Siebke K, Laisk A, Oja V, Kiirats O, Raschke K, Heber U (1990) Control of photosynthesis in leaves as revealed by rapid gas exchange and measurements of the assimilatory force F a. Planta 182:513–522
Siggel U (1974) The control of electron transport by two pH-sensitive sites. In: Avron M (ed) Proc. 3rd Internat. Congr. on Photosynth. Elsevier, Amsterdam, pp 645–654
Stitt M (1987) Fructose 2,6-bisphosphate and plant carbohydrate metabolism. Plant Physiol 84:201–204
Vallon O, Bulte L, Dainese P, Olive J, Bassi R, Wollman F-A (1991) Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions. Proc Natl Acad Sci USA 88:8262–8266
Viil J, Laisk A, Oja V, Pärnik T (1972) Positive influence of oxygen on photosynthesis. Doklady AN SSSR (Proc Acad Sci USSR) 204(5):1269–1271 (in Russian)
Viil J, Laisk A, Oja V, Pärnik T (1977) Enchancement of photosynthesis caused by oxygen under saturating irradiance and high CO2 concentrations. Photosynthetica 11(3):251–259
Winter H, Robinson DG, Heldt HW (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191:180–190
Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193:530–535
Yin X, Harbinson J, Struk PC (2006) Mathematical review of literature to assess alternative electron transports and interphotosystem excitation partitioning of steady-state C3 photosynthesis under limiting light. Plant Cell Environ doi: 10.1111/j.1365-3040.2006.01554.x: 1–12 (in press)
Yin X, van Oijen M, Schapendonk AHCM (2004) Extension of a biochemical model for the generalized stoichiometry of electron transport limited C3 photosynthesis. Plant Cell Environ 27:1211–1222
Zhu X-G, Govindjee, Baker NR, deSturler E, Ort DR, Long SP (2005) Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with photosystem II. Planta 223:114–133
Acknowledgements
This work was supported by Targeted Financing Theme 0182535s03 from Estonian Government and by Grants 6607 and 6611 from Estonian Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1 Model constants and denotations
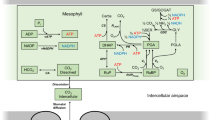
Acronyms and denotations used in Fig. 1 and in the text beyond model parameters and denotations: A, net CO2 assimilation rates; ADPGlu, ADP-glucose; C a, C c, CO2 concentration, ambient and at Rubisco sites, respectively; Cyt f, cytochrome f; DHAP, dihydroxyacetone phosphate; E4P, erythrose 4-phosphate; FBP, fructose 1,6-bisphosphate; F2,6BP, fructose 2,6-bisphosphate; Fd, ferredoxin; F6P, fructose 6-phosphate; GAP, glyceraldehyde phosphate; G6P, glucose 6-phosphate; G1P, glucose 1-phosphate; MDH, malate dehydrogenase; NPQ, non-photochemical quenching; OEC, oxygen evolving complex; OP, inorganic phosphate; OPOP, pyrophosphate; PFD, photon flux density and PAD, photon absorption density, mol quanta m−2 s−1; PGA, 3-phosophoglycerate; PQ, plastoquinone; PS2, photosystem II; PC, plastocyanin; PGl, phosphoglycolate; P700, donor pigment of photosystem I; R p, rate of production of CO2 by photorespiration; R5P, ribose 5-phosphate; Ru5P, ribulose 5-phosphate; RuBP, ribulose 1,5-bisphosphate; SBP, seduheptulose 1,7-bisphosphate; S7P, seduheptulose phosphate; SP, sucrose phosphate; P5P, pentose 5-phosphates; T3P, triose 3-phosphates; UDPG, UDP-glucose.
Rubisco (ribulose 1,5-bisphosphate carboxylase-oxygenase) intermediate complexes: ER, with RuBP; EPP, with two PGA; EPG, with PGA and phosphoglycolate; EP, with one PGA; EOP, with phosphate; Ef, free enzyme.
Below the constants for individual reactions used in calculations are listed. References used as guides were given in the “Model” section. Some adjustments were made to fit the model responses to the kinetic experiments. Denotations here correspond to denotations in the program.
Environmental and general constants
Gas phase diffusion resistance (s mm−1): R gw = 4.0E−1, varied during the light response curve;
Mesophyll diffusion resistance (s mm−1): R md = 3.0E−2;
Leaf temperature: T leaf = 22 °C;
Stromal pH: pHs = 8.04; Cytosolic pH: pHc = 7.8;
Total chlorophyll content: Chl = 3.5E−4 mol m−2;
Distribution of Chl to PSII: C chl2 = 0.48;
Distribution of Chl to PSI: C chl1 = 0.48;
Thylakoid membrane volume, (l mol−1 Chl): C vme = 4.0;
Lumen volume, relative to membrane: C lu = 1.0;
Stroma volume, relative to membrane: C st = 8.0;
Cytosol volume, relative to membrane: C cy = 8.0;
Standard midpoint redox potentials of electron carriers in Volts
E QA = −0.05; E PQ = 0; E Cytf = 0.27; E PC = 0.29; E P700 = 0.365;
E Fd = −0.35; E NADPH = −0.32; E NADH = −0.32 (difference E Fd − E NADPH was adjusted as required to fit the PSI acceptor side closure).
Total pools, mol m −2 of leaf area
PS1 density: PS1T = 1.5E−6;
PSII density: PS2T = 2.0E−6;
Plastoquinone: PQT = 13E−6;
Cytochrome b 6 f CytfT = 1.5E−6;
Plastocyanin: PCT = 4.5E−6;
NADP(H): NADPT = 25E−6;
Adenylates: ADT = 10E−6;
Orthophosphate in chloroplasts OPT = 270E−6;
Orthophosphate in cytosol: OPTc = 400E−6;
(0) Ribulose-bisphosphate carboxylase-oxygenase (Rubisco)
Catalytic site turnover rate: k cat = 3 s−1; K m0(CO2) = 11.5E−6 M; CO2/O2 specificity K sp = 92;
RuBP binding: k 1 = 2.0E+4; k _1 = 9.0E−1; CO2 binding irreversible: k _2 = 0; First PGA dissociation irreversible: k _4 = 0; second PGA dissociation reversible: k _5 = 7.0E+4;
O2 binding irreversible: k _6 = 0; phosphoglycolate dissociation irreversible: k _7 = 0.
Other Rubisco constants were computed as follows:
Total concentration of enzyme E 0T: = V m0/k cat, where V m0 was adjusted as measured;
k 4 = 2V m0/E 0T; k 5 = k 4; k 7 = 0.5k 4; k 2 = V m0/K m0(CO2)/E 0T; k 6 = k 2/K sp.
Reactions in chloroplast stroma (V m in mol m−2 s−1; G(= ΔG ′0 ) in kcal mol−1; K m in M). The V m values of the PGA reduction and RuBP regeneration chain were adjusted each to the metabolic control coefficient of less than 0.05 at the maximum CO2 and light-saturated rate.
(1) Complexed PGA-kinase-GAP-dehydrogenase (PGK-GAPDH): V m1 = 2.0E-3; G 1 = 3;
(3) Aldolase (DHAP + GAP → FBP): V m3 = 4.0E−4;
(4) Fructose-bisphosphate phosphatase: V m4 = 5.0E−5;
(5 and 8) Transketolase, constants given for reverse reaction
V m_5 = 3.0E−3; V m_8 = 1.45E−3; G 5 = 1.4; G 8 = 0.1;
(6) Aldolase (DHAP + E4P → SBP): V m6 = 2.0E−4;
(7) Seduheptulose bisphosphatase: V m7 = 1.0E−4;
(9) Phosphoribulokinase: V m9 = 1.0E−2.
Values for K m and other constants of the CRC, starch, and sucrose synthesis enzymes were the same as in (Laisk and Edwards 2000), except SPS was adjusted to V m28 = 3.3E−6 in order to match the phosphate-limited, CO2 and light-saturated photosynthetic rate in the given leaf (strong metabolic control at CO2 and light saturation, shared with Cyt b 6 f).
(12) ATP synthase: V m12 = 3.0E−4 (corresponds to low metabolic control); G 12 = 7.3; K m12ADP = 3.0E−4; K m12OP = 3.0E−4; K m12ATP = 4.86E−1; HPR = 4.
Electron/proton transport reactions
(40) Rate constant for plastocyanin diffusion: RC 40 = 200 s−1 (low control);
(42) Cytochrome b 6 f: V m42 = 2.8E-4 mol leaf m−2 s−1 (relatively strong control shared with SPS at CO2 saturation); K m42PQ_ = 2.0E−6 mol leaf m−2; HER = 2.0;
(44) Proton leakage rate constant: RC 44 = 0;
(45) Electron transfer from PSII acceptor Q A to plastoquinone: RC 45 = 2.5E+9 (very fast);
(46) NDH-dependent cyclic e− flow from NADPH to PQ:
V m46 = 1.0E−5 mol m−2 s−1 (very slow); K m46NADPH = 1.0E−0006; K m46PQ = 1.0E−6; K m46NADP = 1.0E−6; K m46PQ_ = 1.0E−6;
(47) Malate dehydrogenase-based NADPH-NAD shuttle: V m47 = 30E−6 mol m−2 s−1 (being redox-activated shares the control of the alternative electron flow with the Mehler reaction at the atmospheric O2 concentration); K m47NADPH = 1.0E−6; K m47NAD = 1.0E−4; K m47NADP = 1.0E−6; K m47NADH = 1.0E−4 (here K m in mol m−2).
(49) Mehler type O2 reduction rate constant: RC 49 = 4 s−1;
Kinetics of non-photochemical excitation quenching
(50) pK for q E site protonation: pK 50 = 5.65; rate constant for q E induction RC 50 = 3E−2 s−1; k f = 0.149; k N = 0.603; k I = 0.015.
Redox activation of CRC enzymes
Redox potential of thioredoxin f: E SHf = −0.31 V;
V m of thioredoxin f reduction V m52 = 7.2E−4;
Redox potential of carbon reduction enzyme SH groups: E SHcrc = −0.29 V;
V m of enzyme reduction from thioredoxin f: V m54 = 3.6E−2.
Redox activation control of the malate valve
Redox potential of thioredoxin m: E SHm = −0.315 V;
V m of thioredoxin m reduction V m53 = 3.0E−4;
Redox potential of MDH enzyme SH groups E SHmdh = −0.33 V (adjusted to fit the activation of the alternative electron transport);
V m of enzyme reduction from thioredoxin m: V m55 = 3.0E−4 (also adjusted to fit the time kinetics of the alternative electron flow).
Appendix 2 Budget equations
These are differential budget equations that describe the movement of metabolites through the chain of reactions, the rates of which are given by the concentration-dependent rate equations. One differential equation stands for every metabolite (except for those that are considered to be in equilibrium, where the whole group stands as one single pool). Since correct budgeting of carbon, adenylates and reducing equivalents is the primary prerequisite of such metabolic modeling, we list the complete set of equations here with comments (reaction numbering corresponds to Fig. 1).
Electron–proton transport and ATP synthesis
rates V are in mol e− m−2 s−1, V 46 is NDH;
electron budget for the redox-equilibrated Cytf–PC complex; V 61 is chlororespiratory terminal oxidase, a very slow rate (Eq. 36);
electron budget for the redox-equilibrated PC-P700 complex;
The proton budget is an example how compartment volumes are considered when substances are transported through the compartment boundaries.
the second term in parentheses considers electron consumption in the photorespiratory pathway;
V 19 is ATP consumption for starch synthesis; the last two terms consider ATP consumption in the photorespiratory pathway for glycerate phosphorylation and for ammonia re-assimilation.
Carbon reduction cycle
in the PGA budget the first two terms account for the production of PGA from the Rubisco reaction, the terms in the brackets account for the rebinding of PGA to the free and PGA-containing enzyme, V O/2 accounts for phosphorylated glycerate returning from glycolate pathway and V PGAout is the flux of PGA through the phosphate translocator.
V 3 and V 6 are aldolase, V 5 and V 8 are transketolase rates; the last term is phosphate translocator;
V 19 is the rate of starch synthesis pathway;
Starch synthesis pathway
Reactions in cytosol, sucrose synthesis
the last term considers PGA consumption for mitochondrial respiration;
A rev denotes the rate of ‘internal’ carbon feeding into the triosephosphate pool, probably due to glycolysis;
dF26BP c /dt = V 31 −V 32; the budget of F2,6BP see Laisk et al. (1989);
Non-photochemical excitation quenching
QH is a quenching site that is immediately protonated, while N q follows the protonation state slowly. Equation 69 adjusts the time kinetics of the non-photochemical quenching.
Ferredoxin-thioredoxin mechanism of enzyme activation
In order to reproduce the relatively slow time kinetics of the process, enzyme activation was modeled with the help of two differential equations, the first for the reduction of a thioredoxin by ferredoxin and the second for the reduction of enzyme SS groups by the thioredoxin:
The driving force is the redox ratio of a compound, denoted RFd −/RFd, RSH f/RSS f, and RSH crc/RSS crc, where the subscript f stands for thioredoxin f and subscript crc for a regulated carbon reduction enzyme (redox potentials were assumed to be the same for all regulated CRC enzymes). Similar equations were used to model the activation state of malate dehydrogenase, activated by thioredoxin m (reaction 53) and MDH (reaction 55). By setting different E m for the sulfhydryl groups of the carbon reduction enzymes and MDH, as well as different V m values, different time kinetics of activation of the CRC enzymes and MDH were adjusted.
This system was computer-integrated using Euler’s method, programmed in Turbo Pascal 7.0. The integration step was made variable between 0 and 20 ms, calculated from the condition that during the step the most stiff (fastest-turnover) pool could change no more than by 0.1%. This guaranteed the stability of the system at practically all transients (although under stiff conditions the integration might virtually stop due to the very short step, run-time errors were avoided).
Rights and permissions
About this article
Cite this article
Laisk, A., Eichelmann, H. & Oja, V. C3 photosynthesis in silico . Photosynth Res 90, 45–66 (2006). https://doi.org/10.1007/s11120-006-9109-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-006-9109-1