Abstract
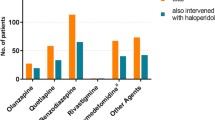
Background Starting in 2007, regulatory agencies strengthened label warnings for intravenous haloperidol. Based on adverse drug reaction (ADR) reports of QT prolongation and torsades de pointes, regulatory agencies recommended the use of continuous telemetry or advising against the intravenous administration in general. Intravenous haloperidol is commonly used as a first line treatment for acute delirium. Consequently, the extended warning has caused uncertainty among health care professionals. Objective The aim of this study is to critically evaluate the WHO global individual case safety report (ICSR) database VigiBase for QT prolongation, torsades and/or cardiac arrest involving intravenous haloperidol compared to other routes of administration and the antipsychotics olanzapine and quetiapine. Method All WHO safety reports (1972–2010) of cardiac reactions associated with haloperidol, quetiapine and olanzapine were evaluated, including dose, route of administration and patient risk factors. Reporting odds ratios for the 3 antipsychotics were calculated. Main outcome measure Number of submitted reports on different antipsychotics. Results The absolute number of ICSR regarding QT prolongation, torsades and/or cardiac arrest were: haloperidol (365 cases), olanzapine (489) and quetiapine (520). Reporting rates of haloperidol did not increase over the last two decades. 32% of the haloperidol cases involved oral, 16.4% intramuscular and 22.7% intravenous administration. The difference of the reporting odds ratios of haloperidol and quetiapine were not statistically significant. Olanzapine was associated with a slightly lower reporting odds ratio. Conclusion While regulatory agencies advise against the use of intravenous haloperidol, review of VigiBase does not reveal that the intravenous route is any more likely to be associated with cardiac adverse events. Furthermore, our results do not demonstrate any additional risk associated with haloperidol when compared with alternative agents. Although pharmacovigilance data does not routinely include a denominator regarding frequency of use, regulatory agencies are currently advising against the use of intravenous haloperidol based on pharmacovigilance, but the number of overall reports is greater for quetiapine and olanzapine when compared to haloperidol. Improved pharmacovigilance approaches are needed to more accurately address the safe, effective use of medicines.
Similar content being viewed by others
References
Food and Drug Administration. FDA alert: Haloperidol (marketed as Haldol, Haldol Decanoate and Haldol Lactate). This alert highlights revisions to the labeling for haloperidol. 2007. Available from: www.fda.gov. Cited 30 Jun 2010.
Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1–20.
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41.
Haldo lV injection (for immediate release) Package Insert. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc.; 2005;rev. 05.2007.
Documed AG Basel. Arzneimittel-Kompendium der Schweiz. 2010. No date. Available from: www.kompendium.ch. Cited 12 Feb 2010.
CPS Compendium of Pharmaceuticals and Specialties, the Canadian drug reference for health professionals. The Canadian Pharmacists’ Association; 2007.
VIDAL—l’information sur les produits de sante. 2008. No date. Available from: www.vidal.fr. Cited 12 Feb 2010.
Rote Liste Service GmbH Frankfurt am Main. Rote Liste Deutschland. 2008. No date. Available from: www.rote-liste.de. Cited 12 Feb 2010.
BNF British National Formulary 2007, compendium of pharmaceuticals and specialties of the UK. 2007. Available from: www.bnf.org. Cited 28 Feb 2009.
Haldol iniettabile—ufficiale monografia italiana. 2009. Availble from: www.informatorefarmaceutico.it. Cited 28 Feb 2009.
Meyer-Massetti C, Cheng CM, Sharpe BA, Meier CR, Guglielmo BJ. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5:E8–16.
Arzneimittelkommission der Deutschen Ärzteschaft AkdÄ. Information über die Änderung der bisher empfohlenen Applikationswege von Haldol-Janssen Injektionswege. Drug safety mail [098]. 2010. Available from: http://www.akdae.de/Arzneimittelsicherheit/DSM/Archiv/2010-098.html. Cited 7 May 2010.
Lindquist M, Edwards IR. The WHO Programme for International Drug Monitoring, its database, and the technical support of the Uppsala Monitoring Center. J Rheumatol. 2001;28:1180–7.
Egberts AC, Meyboom RH, De Koning FH, Bakker A, Leufkens HG. Non-puerperal lactation associated with antidepressant drug use. Br J Clin Pharmacol. 1997;44:277–81.
van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11:3–10.
Ozeki Y, Fujii K, Kurimoto N, Yamada N, Okawa M, Aoki T, et al. QTc prolongation and antipsychotic medications in a sample of 1017 patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:401–5.
Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–9.
Sipahimalani A, Masand PS. Olanzapine in the treatment of delirium. Psychosomatics. 1998;39:422–30.
Schwartz TL, Masand PS. Treatment of Delirium With Quetiapine. Prim Care Companion J Clin Psychiatry. 2000;2:10–2.
Czekalla J, Kollack-Walker S, Beasley CM Jr. Cardiac safety parameters of olanzapine: comparison with other atypical and typical antipsychotics. J Clin Psychiatry. 2001;62(Suppl 2):35–40.
Agelink MW, Majewski T, Wurthmann C, Lukas K, Ullrich H, Linka T, et al. Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. J Clin Psychopharmacol. 2001;21:8–13.
Bosch RF, Baumbach A, Bitzer M, Erley CM. Intoxication with olanzapine. Am J Psychiatry. 2000;157:304–5.
Cohen H, Loewenthal U, Matar M, Kotler M. Association of autonomic dysfunction and clozapine. Heart rate variability and risk for sudden death in patients with schizophrenia on long-term psychotropic medication. Br J Psychiatry. 2001;179:167–71.
Bouman WP, Pinner G. Use of atypical antipsychotic drugs in old age psychiatry. Adv Psychiatr Treat. 2002;8:49–58.
Rea RS, Battistone S, Fong JJ, Devlin JW. Atypical antipsychotics versus haloperidol for treatment of delirium in acutely ill patients. Pharmacotherapy. 2007;27:588–94.
Torres R, Mittal D, Kennedy R. Use of quetiapine in delirium: case reports. Psychosomatics. 2001;42:347–9.
ARIZONA CERT, Arizona Center for Education and Research on Therapeutics. No date. Available from: www.azcert.org. Cited 18 June 2011.
Janicak PG, Javaid JI, Sharma RP, Leach A, Dowd S, Davis JM. A two-phase, double-blind randomized study of three haloperidol plasma levels for acute psychosis with reassignment of initial non-responders. Acta Psychiatr Scand. 1997;95:343–50.
US Food and Drug Administration. FDA Public Health Advisory: deaths with antipsychotics in elderly patients with behavioural disturbances. 2005. Available from: http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/UCM053171. Cited 22 Feb 2010.
US Food and Drug Administration. FDA requests boxed warnings on older class of antipsychotic drugs. FDA news release. 2008. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116912.htm. Cited 22 Feb 2010.
Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–36.
Neil W, Curran S, Wattis J. Antipsychotic prescribing in older people. Age Ageing. 2003;32:475–83.
Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore). 2003;82:282–90.
Acknowledgments
The authors would like to thank Louise Mallet, Pharm D, Certified Geriatric Pharmacist, Professor of Clinical Pharmacy at the University of Montreal and Clinical Pharmacist in Geriatrics at the Royal Victoria Hospital in Montreal for the provision of vast background knowledge on geriatric delirium.
Funding
None.
Conflicts of interest
The authors don’t have any conflict of interest whatsoever to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Carla Meyer-Massetti and Simone Vaerini are co-first-authors.
The data on which this work is based were obtained from the WHO Collaborating Centre for International Drug Monitoring, Uppsala, Sweden. The information contained in this work is not homogeneous at least with respect to origin or likelihood that the pharmaceutical product caused the adverse reaction. The information contained in this work does not represent the opinion of the World Health Organization.
Rights and permissions
About this article
Cite this article
Meyer-Massetti, C., Vaerini, S., Rätz Bravo, A.E. et al. Comparative safety of antipsychotics in the WHO pharmacovigilance database: the haloperidol case. Int J Clin Pharm 33, 806–814 (2011). https://doi.org/10.1007/s11096-011-9541-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-011-9541-y