Abstract
Purpose
To improve nanoliposomal-doxorubicin (DoxNL) delivery in tumor cells using liposome membrane-incorporated short-chain sphingolipids (SCS) with selective membrane-permeabilizing properties. DoxNL bilayers contained synthetic short-chain derivatives of known membrane microdomain-forming sphingolipids; C8-glucosylceramide (C8-GluCer), C8-galactosylceramide (C8-GalCer) or C8-lactosylceramide (C8-LacCer).
Methods
DoxNL enriched with C8-GluCer or C8-GalCer were developed, optimized and characterized with regard to size, stability and drug retention. In vitro cytotoxic activity was studied in a panel of human tumor cell lines and normal cells. Intracellular Dox delivery was measured by flow cytometry and visualized by fluorescence microscopy. For a further understanding of the involved drug delivery mechanism confocal microscopy studies addressed the cellular fate of the nanoliposomes, the SCS and Dox in living cells.
Results
C8-LacCer-DoxNL aggregated upon Dox loading. In tumor cell lines SCS-DoxNL with C8-GluCer or C8-GalCer demonstrated strongly increased Dox delivery and cytotoxicity compared to standard DoxNL. Surprisingly, this effect was much less pronounced in normal cells. Nanoliposomes were not internalized, SCS however transfered from the nanoliposomal bilayer to the cell membrane and preceded cellular uptake and subsequent nuclear localization of Dox.
Conclusion
C8-GluCer or C8-GalCer incorporated in DoxNL selectively improved intracellular drug delivery upon transfer to tumor cell membranes by local enhancement of cell membrane permeability.








Similar content being viewed by others
Abbreviations
- C8-GluCer:
-
C8-glucosylceramide
- C8-GalCer:
-
C8-galactosylceramide
- C8-LacCer:
-
C8-lactosylceramide
- D:PL ratio:
-
Drug to phospholipid initial mass ratio
- Dox:
-
Doxorubicin
- FCS:
-
Fetal calf serum
- HSPC:
-
Hydrogenated soy phosphatidylcholine
- DSPE-PEG2000 :
-
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-PEG2000
- MIF:
-
Mean of fluorescence intensity
- NBD PE:
-
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl)
- NL:
-
Nanoliposomes
- pdi:
-
Polydispersity index
- SCS:
-
Short chain sphingolipids
- SEM:
-
Standard error of the mean
- TCMM:
-
Tumor cell membrane modulation
References
Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal dox: review of animal and human studies. Clin Pharmacokinet. 2003;42(5):419–36.
Koning GA, Krijger GC. Targeted multifunctional lipid based nanocarriers for image guided drug delivery. Anti Cancer Agents Med Chem. 2007;7(4):425–40.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Contr Release. 2000;65(1–2):271–84.
Mayer D, Nayar R, Thies RL, Boman NL, Cullis PR, Bally MB. Identification of vesicle properties that enhance the antitumor activity of liposomal vincristine against murine L1210 leukemia. Cancer Chemother Pharmacol. 1993;33(1):17–24.
Mayer LD, Bally MB, Cullis PR, Wilson SL, Emerman JT. Comparison of free and liposome encapsulated Dox tumor drug uptake and antitumor efficacy in the SC115 murine mammary tumor. Cancer Lett. 1999;53(2–3):183–90.
Veldman RJ, Zerp S, van Blitterswijk WJ, Verheij M. N-hexanoyl-sphingomyelin potentiates in vitro Dox cytotoxicity by enhancing its cellular influx. Br J Cancer. 2004;90(4):917–25.
Veldman RJ, Koning GA, van Hell A, Zerp S, Vink SR, Storm G, et al. Coformulated N-Octanoyl-glucosylceramide improves cellular delivery and cytotoxicity of liposomal Dox. J Pharmacol Exp Ther. 2005;315(2):704–10.
van Lummel M, van Blitterswijk WJ, Vink SR, Veldman RJ, van der Valk MA, Schipper D, et al. Enriching lipid nanovesicles with short-chain glucosylceramide improves Dox delivery and efficacy in solid tumors. FASEB J. 2011;25(10):280–9.
Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9(1):7–14.
Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225.
Masserini M, Ravasi D. Role of sphingolipids in the biogenesis of membrane domains. Biochim Biophys Acta. 2001;1532(3):149–61.
Siskind LJ, Colombini M. Sphingosine forms channels in membranes that differ greatly from those by ceramide. J Bioenerg Biomembr. 2005;37(4):227–36.
Bodley A, Liu LF, Israel M, Seshadri R, Koseki Y, Giuliani FC, et al. DNA Topoisomerase II-mediated interaction of Dox and daunorubicin congeners with DNA. Cancer Res. 1989;49(21):5969–78.
Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727–41.
Rahman AM, Yusuf SW, Ewer MS. Anthracycline-induced cardiotoxicity and the cardiac-sparing effect of liposomal formulation. Int J Nanomedicine. 2007;2(4):567–83.
O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. CAELYX Breast Cancer Study Group, Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal Dox HCl (CAELYX/Doxil®) versus conventional Dox for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–9.
Torchilin VP, Omelyanenko VG, Papisov MI, Bogdanov Jr AA, Trubetskoy VS, Herron JN, et al. Poly (ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim Biophys Acta. 1994;1195(1):11–20.
Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, et al. Pegylated-liposomal Dox versus Dox, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445–51.
Tejada-Berges T, Granai CO, Gordinier M, Gajewski W. Caelyx/Doxil for the treatment of metastatic ovarian and breast cancer. Expert Rev Anticancer Ther. 2002;2(2):143–50.
Hussein MA, Anderson KC. Role of liposomal anthracyclines in the treatment of multiple myeloma. Semin Oncol. 2006;31(6 Suppl 13):147–60.
Alberts DS, Liu PY, Wilczynski SP, Clouser MC, Lopez AM, Michelin DP, et al. Randomized trial of pegylated liposomal Dox (PLD) plus carboplatin versus carboplatin in platinum-sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy (Southwest Oncology Group Protocol S0200). Gynecol Oncol. 2008;108(1):90–4.
Seynhaeve AL, Hoving S, Schipper D, Vermeulen CE, Wiel-Ambagtsheer G, van Tiel ST, et al. Tumor necrosis factor a mediates homogeneous distribution of liposomes in murine melanoma that contributes to a better tumor response. Cancer Res. 2007;67(19):9455–62.
Laginha KM, Verwoert S, Charrois GJ, Allen TM. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin Cancer Res. 2005;11(19 Pt 1):6944–9.
Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2001;37(7):870–7.
Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151(2):201–15.
Rouser G, Fkeischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5(5):494–6.
Li X, Hirsh DJ, Cabral-Lilly D, Zirkel A, Gruner SM, Janoff AS, et al. Dox physical state in solution and inside liposomes loaded via a pH gradient. Biochim Biophys Acta. 1998;1415(1):23–40.
Maurer-Spurej E, Wong KF, Maurer N, Fenske DB, Cullis PR. Factors influencing uptake and retention of amino-containing drugs in large unilamellar vesicles exhibiting transmembrane pH gradients. Biochim Biophys Acta. 1999;1416(1–2):1–10.
Jaffe EA, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52(11):2745–56.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–12.
Zolnik BS, Stern ST, Kaiser JM, Heakal Y, Clogston JD, Kester M, et al. Rapid distribution of liposomal short chain ceramide in vitro and in vivo. Drug Metab Dispos. 2008;36(8):1709–15.
Charrois GJ, Allen TM. Rate of biodistribution of STEALTH liposomes to tumor and skin: influence of liposome diameter and implications for toxicity and therapeutic activity. Biochim Biophys Acta. 2003;1609(1):102–8.
Bandekar A, Karve S, Chang M, Mu Q, Rotolo J, Sofou S. Antitumor efficacy following the intracellular and interstitial release of liposomal doxorubicin. Biomaterials. 2012;33:4345–52.
Forssen EA, Coulter DM, Proffitt RT. Selective in vivo localization of daunorubicin small unilamellar vesicles in solid tumors. Cancer Res. 1992;52(12):3255–61.
Gabizon AA. Selective tumor localization and improved therapeutic index of anthracyclines encapsulated in long-circulating liposomes. Cancer Res. 1992;52(4):891–6.
Perez-Soler R, Ling YH, Zou Y, Priebe W. Cellular pharmacology of the partially non-cross-resistant anthracycline annamycin entrapped in liposomes in KB and KB-V1 cells. Cancer Chemother Pharmacol. 1994;34(2):109–18.
Koning GA, Morselt HW, Velinova MJ, Gorter A, Allen TM, Zalipsky S, Et al.,Selective transfer of a lipophilic prodrug of 5-fluorodeoxyuridine from immunoliposomes to colon cancer cells. Biochim Biophys Acta. 1999;1420(1–2):153–67.
Koning GA, Gorter A, Scherphof GL, Kamps JA. Antiproliferative effect of immunoliposomes containing 5-fluorodeoxyuridine-dipalmitate on colon cancer cells. Br J Cancer. 1999;80(11):1718–25.
Cyrus T, Winter PM, Caruthers SD, Wickline SA, Lanza GM. Magnetic resonance nanoparticles for cardiovascular molecular imaging and therapy. Expert Rev Cardiovasc Ther. 2005;3(4):705–15.
Pinnaduwage P, Huang L. Stable Target-Sensitive immunoliposomes. Biochemistry. 1992;31:2850–5.
Acknowledgments and Disclosures
This work was financed by the Dutch Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Movie 1
Localization of NBD-PE labeled C8-GalCer-DoxNL (green) and Dox (red) by confocal microscopy. BLM melanoma cell line was treated with 5 μM Dox formulated in NBD labelled- C8-GalCer-DoxNL and fluorescence of SCS and Dox was followed in time for a total of 40 min (AVI 45116 kb)
Supplemental Figure 1aSupplemental Figure 1b
Stability at 37°C in the presence of 10%FCS of enriched C8-GluCer-DoxNL (■) was measured for 24 h (A) and enriched C8-GalCer-DoxNL (▲) for 15 h (B). Measurements were based on doxorubicin fluorescence following time and were performed continuously. Both enriched-DoxNL presented a better stability profile than non enriched-DoxNL (●) (JPEG 16 kb)
(JPEG 16 kb)
High Resolution Image
(TIFF 2211 kb)
High Resolution Image
(TIFF 2211 kb)
Supplemental Figure 2a
In vitro drug efficacy toward tumor cells (BLM, Mel57 melanoma, MCF7 and SKBR3 breast carcinoma, Panc1 and ASPC1 pancreatic carcinoma) and non-tumor cells (HUVEC and 3 T3). (A) Cells were treated with Doxil (●), non enriched-DoxNL (■), 10% C8-GluCer-DoxNL (▲) and 10% C8-GalCer-DoxNL (▼) for 24 h at 37°C. Cell survival was quantified by colorimetric SRB assay. (B) Cellular morphology was analyzed by microscopy. Values represent the mean ± SEM of at least 3 experiments (JPEG 132 kb)
High Resolution Image
(TIFF 138 kb)
Supplemental Figure 2b
(DOC 273 kb)
Supplemental Figure 3
Co-localization of NBD-PE labeled liposomes (green) and Dox (red) by Confocal Microscopy. After nucleus staining with Hoechst (blue), BLM melanoma cell line was treated with 10 μM Dox in form of C8-GluCer-DoxNL (A) and C8-GalCer-DoxNL (B). ASPC1 pancreatic carcinoma cells were equally treated with 10 μM Dox in form of C8-GluCer-DoxNL (C) and C8-GalCer-DoxNL (D). After treatment cells were incubated for 4 h at 37°C. Nuclear and cytoplasmatic drug uptake was analysed (PPT 1299 kb)
Supplemental Figure 4
Intracellular Dox uptake after treatment with DoxNL, 1 μM was quantified by flow cytometry in BLM melanoma, MCF7 breast carcinoma and ASPC1 pancreatic carcinoma (A). Doxorubicin was formulated in non enriched-DoxNL (open), 10% C8-GluCer-DoxNL (dark grey) and 10% C8-GalCer-DoxNL (black). Fluorescence of non treated cells was measured as a control (bright grey). At least three independent experiments were performed and values represent the mean ± SEM; *, P < 0.001; **, P < 0.01 and #, P < 0.05 versus non enriched-DoxNL at the same time point. (B) Overview of Dox uptake by 3 different tumor cell lines incubated with 1 μM C8-GluCer or C8-GalCer-DoxNL after 4 h of incubation. C8-GluCer seemed to increase Dox uptake in BLM (open) cells when compared to MCF7 (grey) and ASPC1 (black) cells. C8-GalCer-DoxNL gives similar results in all three cell lines. (JPEG 3.44 mb)
High Resolution Image
(TIFF 12946 kb)
Supplemental Table 1
In vitro IC50 values toward tumor cells BLM, melanoma and SKBR3, breast carcinoma, after treatment with C8-GluCer-DoxNL following different drug to phospholipid ratios: 0.25:1; 0.2:1; 0.15:1; 0.1:1 (w/w) (DOC 30.5 kb)
Supplemental Table 2
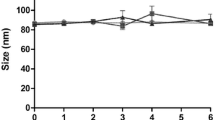
Stability at 4°C after 6 months of different glycoceramide-enriched liposomes in a drug to phospholipid ratios of 0.1:1 (w/w). Size and pdi were analyzed. (DOC 30 kb)
Supplemental Table 3
In vitro IC50 values of doxorubicin in different tumor cell lines, BLM, melanoma, MCF7 breast carcinoma and ASPC, pancreatic carcinoma, following different densities for C8-GalCer-DoxNL (DOC 34 kb)
Rights and permissions
About this article
Cite this article
Pedrosa, L.R.C., van Hell, A., Süss, R. et al. Improving Intracellular Doxorubicin Delivery Through Nanoliposomes Equipped with Selective Tumor Cell Membrane Permeabilizing Short-Chain Sphingolipids. Pharm Res 30, 1883–1895 (2013). https://doi.org/10.1007/s11095-013-1031-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1031-6