Abstract
To investigate the possible interplanetary transfer of life, numerous exposure experiments have been carried out on various microbes in space since the 1960s. In the Tanpopo mission, we have proposed to carry out experiments on capture and space exposure of microbes at the Exposure Facility of the Japanese Experimental Module of the International Space Station (ISS). Microbial candidates for the exposure experiments in space include Deinococcus spp.: Deinococcus radiodurans, D. aerius and D. aetherius. In this paper, we have examined the survivability of Deinococcus spp. under the environmental conditions in ISS in orbit (i.e., long exposure to heavy-ion beams, temperature cycles, vacuum and UV irradiation). A One-year dose of heavy-ion beam irradiation did not affect the viability of Deinococcus spp. within the detection limit. Vacuum (10−1 Pa) also had little effect on the cell viability. Experiments to test the effects of changes in temperature from 80 °C to −80 °C in 90 min (±80 °C/90 min cycle) or from 60 °C to −60 °C in 90 min (±60 °C/90 min cycle) on cell viability revealed that the survival rate decreased severely by the ±80 °C/90 min temperature cycle. Exposure of various thicknesses of deinococcal cell aggregates to UV radiation (172 nm and 254 nm, respectively) revealed that a few hundred micrometer thick aggregate of deinococcal cells would be able to withstand the solar UV radiation on ISS for 1 year. We concluded that aggregated deinococcal cells will survive the yearlong exposure experiments. We propose that microbial cells can aggregate as an ark for the interplanetary transfer of microbes, and we named it ‘massapanspermia’.
Similar content being viewed by others
Introduction
The viability of life in space and the possibility of transfer of life between the Earth and extraterrestrial bodies have been actively pursued as research targets in various areas including astronomy, cosmology, planetary sciences, and biology. This transfer process is called panspermia, which was proposed by Arrhenius (1903). He proposed that the interplanetary transfer of single spores is propelled by radiation pressure from the Sun.
To evaluate the panspermia hypothesis, feasibility of natural interplanetary exchange of microbes inside rocks as the result of natural impacts, a process which is known as ‘lithopanspermia’ or ‘transpermia’ (Mileikowsky et al. 2000; Nicholson et al. 2000; Clark 2001; Horneck et al. 2002; Zagorski 2007; Fajardo-Cavazos et al. 2007; Cockell 2008; Nicholson 2009; Onofri et al. 2012), has been tested both theoretically and experimentally. The scenario of lithopanspermia consists of several phases: rocks ejected from a certain planet (e. g. the Earth or Mars), transport of microbes through the space for a long period, and finally, landing on another planet (Gladman et al. 1996; Mileikowsky et al. 2000; Horneck et al. 2008; Nicholson 2009).
Since the 1960s, various space exposure experiments have been conducted to determine the survivability of microbes and fungi during the transfer in space. Accordingly, various types of microbes have been exposed to selected combined space conditions outside the Earth’s magnetic field (Apollo 16) or in low Earth orbit (LEO): in onboard missions of Spacelab 1, Spacelab D2, ERA on EURECA, LDEF and BIOPAN on FOTON as well as in EXPOSE-E, EXPOSE-R, BIORISK and EXPOSE-R which is in progress on the International Space Station (ISS) (Baranov et al. 2009; Horneck et al. 2010; Olsson-Francis and Cockell 2010; Rabbow et al. 2012; de Vera et al. 2012).
Environmental parameters in LEO—such as extreme vacuum (10−4 ~ 10−6 Pa), intense solar ultraviolet (UV) radiation, various components of cosmic radiation, and high and low temperatures—affect the genetic stability of microbes (Mennigmann 1989). For example, spores of Bacillus subtilis in a multi-layer survived under solar UV radiation for about 6 years though all the spores of B. subtilis in a monolayer were killed (Horneck 1993; Horneck et al. 1994). The result suggested that microbe spores might survive for a long period if the spores are shielded from intense solar radiation (Horneck et al. 2001).
As described in Yamagishi et al. (2008), we have proposed to conduct experiments on capture and space exposure of microbes at the Exposure Facility of Japanese Experimental Module (JEM) of ISS (Tanpopo mission). In this mission, we are going to expose various microbes in space including the deinococcal species. Deinococci are known to be extremely tolerant against ionizing radiation, UV, desiccation and oxidative stress (Anderson et al. 1956; Battista 1997; Daly 2009; Slade and Radman 2011). In fact, D. radiodurans has been tested in space in EURECA mission (Dose et al. 1995). When the cells were exposed to 4 × 105 kJ/m2 light (175 nm to 340 nm) under argon or space vacuum, they did not survive the mission. When a single layer of D. radiodurans cells (108 cells) was exposed to extreme UV (λ = 30.4 nm) radiation and space vacuum during the rocket flight for few minutes or seconds, the exposure to extreme UV radiation decreased their survival by an extra order of magnitude below their desiccation tolerance (Saffary et al. 2002).
Some deinococcal species, such as Deinococcus aerius and Deinococcus aetherius, have been isolated from the high atmosphere (Yang et al. 2009a, 2010). Among these two species, the D. aerius exhibited resistance to γ ray irradiation that was similar to that of the D. radiodurans, whereas the D. aetherius exhibited higher resistance to gamma-ray irradiation than the D. radiodurans. These species also show high resistance to UV radiation (Yang et al. 2008). Thus, these bacteria might be able to survive in the space environment.
In this study, we have examined the survivability of three deinococcal species (D. radiodurans, D. aerius, and D. aetherius) to evaluate whether they are suitable candidates for the space exposure experiments on ISS.
Materials and Methods
Strains and Cell Culture
D. radiodurans strain R1 (ATCC 13939) was purchased from American Type Culture Collection. D. radiodurans was cultured overnight in mTGE medium (1 % Bacto tryptone, 0.6 % beef extract, 0.2 % glucose) at 30 °C in an incubator with shaking at 150 rpm until it reached the stationary phase. D. aerius strain TR0125, isolated from the high atmosphere (Yang et al. 2009a), was cultured for about 7 days in mTGE medium at 30 °C with shaking at 150 rpm to stationary phase. D. aetherius strain ST0316, isolated from the lower stratosphere (Yang et al. 2010), was cultured at 30 °C on mTGE agar plates supplemented with 1 % glycerol for about 2 weeks, as this strain hardly grows in a liquid medium.
Sample Preparation
D. radiodurans and D. aerius cells were collected from their respective liquid cultures by centrifugation at 3,000 rpm for 10 min at 4 °C. D. aetherius cells were collected from the upper parts of the colonies growing on the plate using a platinum loop. These cells were washed with 10 mM potassium phosphate buffer (PB: pH 7.0) and the cells were collected by centrifugation at 3,000 rpm for 10 min at 4 °C. This process was repeated three times. The optical density (OD590nm) of each cell suspension was measured using a spectrophotometer and the measured optical density was used to estimate cell concentration. Calibration curves are shown in Fig. 1.
Heavy-ion Beam Irradiation
Heavy-ion beam irradiation was performed at the Heavy Ion Medical Accelerator at Chiba (HIMAC) in the National Institute for Radiological Science. Helium (He) ion (150 MeV/u, 2.2 keV/μm) and Argon (Ar) ion (500 MeV/u, 90 keV/μm) beams were used for irradiating the cells. For this purpose, each cell suspension (20 μl) was placed in a test tube (1.3 cm diameter, 10 cm length and 8 mm inner diameter, IWAKI). The samples were air-dried for 18 h at room temperature. The amount of the sample, 20 μl, was sufficient to obtain reproducible results, comparing the larger volume. After irradiation, cells were resuspended in 200 μl of 10 mM PB. The heavy-ion beam was irradiated on deinococcal cells at room temperature. The cell suspension was serially diluted with PB, and the diluted cells were spread on mTGE agar plates. The plates were incubated at 30 °C for 3 days, 7 days, and 2 weeks for D. radiodurans, D. aerius, and D. aetherius, respectively. Control samples were not irradiated but treated, as is the case with irradiated sample.
Temperature Cycles and Vacuum
Aluminum plates containing cylindrical wells (1.5 mm diameter, 2 mm depth) with flat floor were manufactured as sample holders (Fig. 2). The aluminum plate is made of duralumin (JIS: A2024-T3), and surface of the plates were anodized. The aluminum plate was dry-heat sterilized at 180 °C for 4 h. Aliquots (3 μl) of cell suspension containing approximately 105 to 108 cells/ml were applied to and dried in each well three times with appropriate time intervals, and subsequently the cell aggregate was further air-dried for 18 h at room temperature. The thermal cycle device (Fig. 3a) was used for the thermal cycle experiment as described in Takahashi et al. (2011). Experimental conditions were as follows. Temperature was changed from 80 °C to −80 °C or from 60 °C to −60 °C in a 90 min cycle. A typical temperature profile is shown in Fig. 3b. Each thermal cycle consisted of cooling for 20 min, stopping of the machine for 25 min, followed by heating for 20 min, and stopping of the machine for 25 min, in total 90 min. All temperature cycle experiments were performed under vacuum (<10−1 Pa). Aluminum plates were retrieved after 0, 1, 112, 224 or 448 cycles. In vacuum exposure experiments, Deinococcus spp. were exposed to vacuum (<10−1 Pa) for 0, 7, 14 or 28 days. After the treatment, dried cells in each well were resuspended and recovered by adding 3 μl of PB to each well. The recovery process was repeated for 5 times by adding PB to the well, and the recovered suspensions were combined and used for colony counting. In temperature cycle and vacuum experiment, for the control sample, air-dried cells were resuspended on the day 0 of the temperature cycle and vacuum experiments. The cells were analyzed using the same method described above.
a Schematic illustration of the workings of the thermal cycle machine. b Profiles of temperature changes. The plot containing open circles shows temperature changes from 80 °C to −80 °C per 90 min (Takahashi et al. 2011). The plot containing open squares shows temperature changes from 60 °C to −60 °C per 90 min
UV Exposure
Aluminum plates with wells described above were used for UV irradiation experiment (Fig. 2). To know how many cells are needed to fill up the well, aliquots of the cell suspension were dropped into the wells and air-dried. This process was repeated until the well was completely filled with air-dried cells. The cells were recovered from each well by using PB. Recovered cells were then used for colony counting as described above. The number of air-dried D. radiodurans, D. aerius, and D. aetherius cells needed for filling up the well (2 mm) was approximately 8.4 × 108, 6.1 × 108, and 7.0 × 108 cells, respectively.
Monochromatic UV light (VUV172 nm or UVC254 nm) was used to irradiate deinococcal cells under vacuum (approximately 10−2 to 10−3 Pa). For VUV172 nm irradiation, monochromatic light (172 ± 7 nm) from a head-on type lamp (model UER20H-172, Ushio Corp.) equipped with an MgF2 window at the lamp end was used (Hirose et al. 2002). For UVC254 nm irradiation, a mercury lamp was used. A sample plate was placed on a supporter in the vacuum chamber at a fixed distance from the lamp. The UV intensity at this distance was measured using a photodiode before and after each irradiation experiment. A control sample plate covered with aluminum foil was placed in the chamber away from irradiation area.
When the irradiation experiment was initiated, the pressure inside the vacuum chamber was kept at approximately 10−2 to 10−3 Pa. The sample plates were exposed to UV for different periods, up to 17 h and 71.7 h for VUV172 nm and UVC254 nm, respectively. After the irradiation, cells were recovered from the wells, serially diluted, spread on the agar plates for colony counting as described above. Surviving fraction was calculated as the ratio of counted cells from the irradiated sample and the respective mock-irradiated control sample that was placed inside the UV chamber under dark.
Survival Curve Fitting for UV-Irradiated Samples
The nonlinear relationship between the survival rates of the Deinococcus spp. against VUV172 nm and UVC254 nm irradiation was determined by curve fitting. When the deinococcal cells were irradiated with UV, the intensity of UV at the surface of cells was defined as I 0 and the intensity of UV that affecting the cells was defined as I. I depends on the depth (thickness) of cells d and the absorption coefficient α (μm−1) and can be described using the Beer-Lambert’s law (Eq. 1).
The energy E of UV irradiation at depth (thickness) d was calculated using I multiplied by time t (Eq. 2).
The survival rate s 1 of the layer of cells at depth d depended on the energy E and the survivability fraction β (m2/J) (Eq. 3). The survivability fraction β is proportional to 1/D10, where D10 is the radiation dose that decreased the number of cells to 10 % of the original number.
We define the survival curve s 2 as the integration of survival rate s 1 (Eq. 3) from the very shallow depth to the specified depth (d):
We fitted s 2 with the experimental data using χ 2 by using solver program of Microsoft Excel and obtained α and β values, as summarized in Table 1.
Results
Heavy-ion Beam
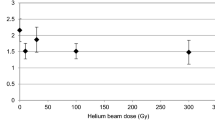
Heavy ions are believed to induce damage to numerous targets within the cells in the space environment. To determine the survivability of deinococcal species against the heavy ion beam irradiation, we irradiated the dehydrated deinococcal cells with helium ions (He; 150 MeV/u) and argon ions (Ar; 500 MeV/u) under normal pressure at room temperature. After irradiation, we calculated the D10 values from the survival curves (Table 2). The D10 values for the He-ions were higher than that for the Ar-ions. Among the three species tested, D. aetherius was most tolerant to heavy-ion beams and D. aerius was least tolerant. Therefore, it was estimated that one-year dose of heavy ion beams have little effect on the viability of Deinococcus spp. (Table 2).
Temperature Cycles
ISS is orbiting around the earth every 90 min. Temperature in ISS becomes high on the sun side, but very low on the shadow side. The temperature conditions to be set in this simulation experiment was determined based on the temperature model calculated for the Tanpopo apparatus placed in the Exposure Facility of JEM on ISS (data not shown). The effects of temperature changes from −80 °C to 80 °C and from −60 °C to 60 °C in a 90 min cycle under 10−1 Pa were examined (Fig. 3b). After 5,840 temperature cycles, which corresponded to a year of exposure to temperature variation, D10 values and survival rates of all three species were determined and the results are summarized in Table 3. D. aetherius was the most resistant and D. aerius was the least resistant species against the temperature cycles. However, the survival rates for the three species following the −60 °C to 60 °C temperature cycle were similar (Fig. 4).
Survival curves of Deinococcus cells in response to temperature changes. Dry cells were subjected to the following temperature cycles: (a) from 80 °C to −80 °C in 90 min per cycle or (b) from 60 °C to −60 °C in 90 min per cycle. Symbols used: circle, D. radiodurans; square, D. aerius; and triangle, D. aetherius. Data shown are expressed as averages ± standard deviations (n = 3)
Vacuum
Deinococcal cells were exposed to continuous vacuum (10−1 Pa) for up to 28 days, and then their survival rates were determined. As shown in Fig. 5, even after 28 days under vacuum, all three species (D. radiodurans, D. aerius and D. aetherius) exhibited high survival rates (86.8 %, 80.1 % and 86.9 %, respectively). The calculated D10 values and survival rates after one-year exposure to vacuum are listed in Table 4. Clearly, the survival rate of Deinococcus spp. after 1 year under vacuum (10−1 Pa) was high, and approximately 1 % to 10 % cells are expected to survive after 1 year.
UV Radiations
We next examined the effect of solar UV radiation on deinococcal cell aggregates of different thicknesses to determine whether the size of the cell aggregate influences the cell survivability. The spectral ranges of UV in the interplanetary space and LEO are different from those on the ground. In the Tanpopo mission, the MgF2 or SiO2 windows will cut off any UV radiation shorter than 110 nm. Thus, in the present study, we exposed deinococcal cells to VUV172 nm or UVC254 nm. Layers of cells were deposited and dehydrated in the wells of the aluminum plate sample holder to form a series of cell aggregates of different thicknesses (thickness between 1 μm and 2,000 μm). The monolayer (thickness: 1 μm) was prepared with less than 4.2 × 105 cells for D. radiodurans, less than 3.1 × 105 cells for D. aerius and less than 3.5 × 105 cells for D. aetherius. The survival curves were drawn using the formula s 2 shown in Figs. 5 and 6.
Survival curves of D. radiodurans (a), D. aerius (b) and D. aetherius (c) following exposure to different doses of VUV172 nm radiation (doses indicated on the right side of each figure) under vacuum. Each survival curve was fitted with the experimental data points as described in Materials and Methods using Eq. 4 and parameters α and β listed in Table 1
The survival of 1-μm (monolayer) cells was decreased even by low doses of UV radiation. Thus, monolayers of D. radiodurans, D. aerius and D. aetherius cells were killed by VUV172 nm doses higher than 288 kJ/m2, 12.2 kJ/m2 and 73.3 kJ/m2, respectively. Similarly, monolayers of D. radiodurans, D. aerius and D. aetherius cells were killed by VUV254 nm doses higher than 8.6 kJ/m2, 1.7 kJ/m2 and 11.1 kJ/m2, respectively. The survival rate, however, increased with the increased thickness of cells (Figs. 6 and 7). Though some part of the monolayer may be aggregated, the layer is thin enough to be killed with very low intensity of UV radiation.
Survival curves of D. radiodurans (a), D. aerius (b) and D. aetherius (c) following exposure to different doses of VUV254 nm radiation (doses indicated on the right side of each figure) under vacuum. Data were processed in the same manner as mentioned in the legend of Fig. 6
We defined T10 as the thickness that enables 10 % survival of cells following one-year exposure to VUV172nm and UVC254 nm. Table 5 summarizes the survival rates of cells in aggregates of various thicknesses (1, 10, 30, 50, 100, 200 and 500 μm) after exposure to VUV172 nm for 1 year. The dose of VUV on ISS orbit was estimated to be 0.12 J/(m2s) for 120 nm to 200 nm radiation (Lean 1991). Thus, the total dose after a year of exposure to VUV172 nm was 3.8 × 103 kJ/m2. The calculated T10 values for D. radiodurans, D. aerius and D. aetherius under 3.8 × 103 kJ/m2 of VUV172 nm exposure were found to be 6 μm, 39 μm and 17 μm, respectively. Table 5 also summarizes the survival rates of cells in aggregates of various thicknesses (1, 50, 100, 200, 500, 1,000 and 2,000 μm) after exposure to VUV254 nm irradiation for 1 year. The dose of UVC on ISS orbit was estimated to be 5.89 J/(m2s) for 200 nm to 280 nm radiation (Lean 1991). Thus, the total dose after a year of exposure to UVC254 nm was 1.9 × 105 kJ/m2. The calculated T10 values for D. radiodurans, D. aerius and D. aetherius under 1.9 × 105 kJ/m2 of UVC254 nm exposure were found to be 46 μm, 413 μm and 327 μm, respectively. The order of resistance of Deinococcus spp. against VUV172nm and UVC254 nm was D. radiodurans, D. aetherius, and then D. aerius. In addition, UVC254 nm was found to be more lethal than VUV172 nm for the Deinococcus spp. However, it should be noted that the surrounding environment of solar panels with varying positions, other ISS structure shads the UV radiation. The dose of UV radiation will be less than the dose of our estimated dose of UV radiation.
Survivability of Deinococcus spp. Against the Effects of Multiple Environmental Factors
In space, harsh environmental factors have damaging effects on microbes, including their DNA, membrane lipids and proteins. We determined the survival rates of Deinococcus spp. following one-year exposure to multiple environmental factors including heavy ions, vacuum (10−1 Pa), γ ray, temperature cycles (±80 °C/90 min cycle and ±60 °C/90 min cycle), and VUV172 nm and UVC254 nm radiation. Survivability of Deinococcus spp. against the effects of multiple environmental factors was calculated by multiplying the survival rates after one-year exposure of heavy-ions, γ ray, temperature cycles, vacuum and UV radiation. Results are summarized in Table 6. The survival rates after one-year of exposure to γ ray for all species were estimated to be 1.0. A sample-holder well (ϕ1.5 mm × 2.0 mm) filled with deinococcal cells was found to contain 8.4 × 108 cells of D. radiodurans, 6.1 × 108 cells of D. aerius, or 7.0 × 108 cells of D. aetherius. It is noteworthy that D. radiodurans and D. aetherius cells survived for 1 year under both test temperature cycles. On the other hand, even though D. aerius cells survived the ±60 °C/90 min cycle, they did not survive the ±80 °C/90 min cycle.
Discussion
In this study, we evaluated the viability of species (D. radiodurans, D. aerius and D. aetherius) after exposure to harsh environmental conditions for a year in the Tanpopo mission. It has been reported earlier that D. radiodurans can not only tolerate high dose of γ ray radiation, but they are also extremely tolerant to other extreme environmental factors (Battista 1997; Blasius et al. 2008; Slade and Radman 2011). D. aerius and D. aetherius, which were isolated from high altitude environment in Japan, also exhibited high resistance to γ and UV radiations (Yang et al. 2009a, 2010).
Effects of Heavy-ion Beam on Deinococcus spp.
The radiation in space mainly consists of two types of radiations: galactic radiation (GCR) and the solar cosmic radiation (SCR) (Hellweg and Baumstark-Khan 2007). The GCR originates in space beyond our Solar system, and it consists of 98 % baryon component and 2 % lepton component. The baryon component is composed of 87 % proton, 12 % helium ion beams (α particles), and the remaining 1 % is heavy ions. The SCR component consists of low energy solar wind particles that constantly flow out of the Sun, and the so-called highly energetic solar particle events, which originate from the magnetically disturbed regions of the Sun, which sporadically emits bursts of energetic charged particles (Wilson et al. 1999). They are predominantly composed of protons with a minor contribution from helium ions (~10 %) and even smaller portions of heavy ions and electrons (1 %).
Effects of heavy-ions on D. radiodurans have been studied before (Dewey 1969; Zimmermann et al. 1994; Kobayashi et al. 1995). The dried cells of D. radiodurans exhibited high resistance against exposure to particles of He, C, Ne, Ar and Ni (Kobayashi et al. 1995). In the interplanetary space, annual dose rate of cosmic ionizing radiation is less than 0.1 Gy/year (Nicholson et al. 2000). In this study, we estimated D10 and survival rate of deinococcal species after 1 Gy exposure (Table 2). The dose of He and Ar ion will not affect the survival of Deinococcus spp. after one-year exposure
Generally, damages induced by ionizing radiation in microbes are various types of DNA damage including double-strand breaks (DSBs), single-strand breaks (SSBs), base modification (AP site), and sugar modification (Zimmermann et al. 1994; Goodhead 1994). DSBs are the most lethal form of DNA damage. It has been shown that D. radiodurans can repair over 100 DSBs in chromosomes during post-irradiation incubation (Dean et al. 1966; Narumi 2003).
D. aetherius has been reported to be more resistant against γ ray (D10 : 8 kGy) than the D. radiodurans (D10 : 6.7 kGy) (Yang et al. 2010). In contrast, D. aerius has exhibited lower resistance against γ ray (D10 : 4.9 kGy) than D. radiodurans (Yang et al. 2009a). The order of resistance to γ ray radiation for the three species was similar to the order of resistance against heavy-ion beam. Interestingly, D. aetherius showed higher tolerance to heavy ions than D. radiodurans and D. aerius (Table 2). These results implied that D. aethterius might have better ability to repair DSBs than D. radiodurans.
Effects of Temperature Cycle on Deinococcus spp.
The temperature of a body in space, which is determined by absorption and emission of energy, depends on its position with respect to the Sun and other orbiting bodies, and also on its surface, size, mass, and albedo (Nicholson et al. 2000; Mileikowsky et al. 2000; Horneck et al. 2002).
A previous study has shown that a 10 min of exposure to 60 °C or 80 °C had no effect on the survival of dried D. radiodurans cells (Bauermeister et al. 2012). These results suggested that the cells could withstand exposure to high temperature (<80 °C) for a short time (Bauermeister et al. 2012). Determining the tolerance of other Deinococcus spp. to changes in temperature cycle is, however, important. In this study, the temperature change tolerance of three Deinococcus spp. was examined. Whereas the ±80 °C temperature cycle severely impaired the survival of Deinococcus spp., the effect was less severe for the ±60 °C temperature cycle (Fig. 4 and Table 3).
The temperature of EXPOSE-E on ISS was reported to fluctuate between 20 °C and 59 °C (Horneck et al. 2012; Rabbow et al. 2012). It is possible that we might have used an overestimated condition in our model for the simulated temperature cycles. If ±60 °C temperature cycles were used during the space experiment, then all the deinococcal species would have survived after 1 year.
Effects of Vacuum on Deinococcus spp.
Dehydration causes severe damage to the cell components—lipid membranes may change from planar bilayers to cylindrical bilayers, and carbohydrates, proteins, and nucleic acids may undergo amino-carbonyl reactions that would result in cross-linking, which would eventually lead to polymerization of biomolecules (Dose et al. 1991; Cox 1993). Based on the results of the effects of vacuum on deinococcal species (Fig. 5 and Table. 4), it is clear the 1 to 10 % of the cells would survive following 1 year exposure to 10−1 Pa vacuum. It has been suggested that the extreme resistance of Deinococci to radiation is a consequence of an adaptation to prolonged desiccation (Mattimore and Battista 1996). Consistent with this notion, D. radiodurans, D. aerius and D. aetherius cells were shown to survive under desiccated condition (Yang et al. 2009b, c; Mattimore and Battista 1996). The observed resistance of Deinococcus spp. against vacuum may be also related to the resistance against desiccation.
Effect of UV Irradiation on Deinococcus spp.
The solar UV wavelengths range from ~10 nm to 400 nm. This entire wavelength range is further classified into UVC (200–280 nm), UVB (280–315 nm), and UVA (315–400 nm). Vacuum UV (VUV) (< 200 nm) can be referred to the UV flux found in the interplanetary space at wavelengths shorter than UVC (Nicholson et al. 2005). From our results, the absorption coefficients α (μm−1) were different as shown in table 1. The absorption coefficient of D. radiodurans was the highest among the three. The absorption at UV region of D. radiodurans may be higher than the other two strains.
Results of the space exposure experiments suggest that among all the space environmental factors, solar UV is most lethal to microbes, and this UV correlated with the absorption wavelength of DNA (Horneck 1993). The DNA directly absorbs the light around UVC (Horneck et al. 1995). However, when shielded against the influx of solar UV, spores of B. subtilis survived in space environment (Horneck et al. 1994). Bacillus spores with clay, soils and glucose as a ‘mixed layers’ and ‘artificial meteorites’ survived for prolonged period in space (Horneck et al. 2001). Determining the tolerance of other Deinococuus spp. to changes in temperature cycle is, however, important. It was found that when microbes were covered with a 1–10 cm thick layer of limonite, they were protected from the solar UV radiation (Silverman et al. 1964; Green et al. 1971; Hagen et al. 1970; de la Vega et al. 2007). Osman et al. (2008) also observed significant survival of spore-forming bacteria isolated from the Atacama Desert from full spectrum Martian UV (200–400 nm) irradiation when shielded by micro soil particles (<60 μm).
The deinococcal isolates from the high atmosphere tended to form cell clumps or aggregates, which was considered to help their survival under UV irradiating environment (Yang et al. 2009c). We have proposed that multi-layered (i.e., aggregated) deinococcal cells may survive in space for a long time (Yamagishi et al. 2008). Consistent with this proposal, we have demonstrated here that D. radiodurans, D. aerius and D. aetherius cells survived after exposure to VUV172 nm and UVC254 nm radiation. Though cells in thin layers or aggregates were killed by UV radiation, large numbers of cells survived the radiation when the cell layer was thick (Figs. 6 and 7). Results of our study suggest that cells near the surface layer were killed by UV, and the layers of killed cells protected the cells underneath from the UV damage. Supposing that the aggregated cells are sphere, the diameter of the cell aggregate that could shield the cells in the inner layer from solar UV radiation is over 200 μm for D. radiodurans, 850 μm for D. aerius, and 700 μm for D. aetherius.
The tolerance of D. radiodurans against UV radiation of shorter wavelength than VUV has also been reported. Thus, when layers of various thicknesses of D. radiodurans cells were exposed to synchrotron light (λ = 121.6 nm), multi-layered cells (7 μm thick) showed higher survival rate than the monolayer cells (Paulino-Lima et al. 2010). Consistent with this observation, we found high survivability of D. radiodurans cells in micrometer-sized aggregates after exposure to VUV172 nm radiation (Fig. 6).
Estimation of Survivability of Deinococcus spp. to Select the Candidates for the Exposure Experiments on Microbes in the Tanpopo Mission
We proposed performing exposure experiments on microbes at the Exposure Facility on ISS-JEM (Yamagishi et al. 2008). As mentioned earlier, Deinococcus spp. are candidate microbes for the Tanpopo mission. In the present study, we have investigated the effects of heavy-ions, γ ray, temperature cycles, vacuum and UV radiation on the survivability of Deinococcus spp. In the Tanpopo mission exposure device, cells that are present deep inside the cell aggregates of few hundred micrometer thickness were shielded from the radiations of VUV172 nm and UVC254 nm. Thus, if deinococcal cells are embedded in the 2-mm-deep wells of the aluminum plates (Fig. 2), D. radiodurans and D. aetherius will survive after exposure experiment for 1 year in the Tanpopo mission (Table 6). Here, the survivability of Deinococcus spp. after 1 year in space was estimated by multiplying the survival rates after one-year exposure of heavy-ions, γ ray, temperature cycles, vacuum and UV radiation. D. aerius cells will be killed when the temperature fluctuation is ±80 °C, but they would survive if the temperature fluctuation is less than ±60 °C. Based on our results, we conclude that Deinococcus spp. could be suitable candidate microbes for exposure experiments.
Microbial Cell-Aggregates as a Form for Interplanetary Transfer of Microbes
Lithopanspermia hypothesis proposes the interplanetary transfer of microbes inside of rocks (reviewed in Nicholson 2009; Horneck et al. 2002, 2010). A few micrometer size of meteorite, which does not have any crack, would be good enough to provide protection against UV radiation (Mileikowsky et al. 2000).
Our results suggest that the aggregated cells of D. radiodurans, D. aerius and D. aetherius are highly resistant to environments of LEO. We would like to emphasize the possible importance of microbial cell-aggregates as an ark for interplanetary transfer of microbes. We call this concept ‘massapanspermia’ (‘Massa’ stands for mass in Latin). The proposed experiment for the Tanpopo mission is intended to test the possibility that this massapanspermia concept might be true. Aggregated cells of D. radiodurans, D. aerius and D. aetherius will be prepared to various thicknesses—from 1 μm (monolayer) to 2,000 μm. After the exposure experiments, we will analyze the viability of Deinococcus spp. to answer whether the aggregated cells would survive the interplanetary journey.
References
Anderson AW, Nordan HC, Cain RF, Parrish G, Duggan D (1956) Studies on a radio-resistant micrococcus. I. Isolation, morphology, cultural characteristics, and resistance to gamma radiation. Food Technol 10:575–578
Arrhenius S (1903) Die verbreitung des lebens im weltenraum. Umschau 7:481–485
Baranov VM, Novikova ND, Polikarpov NA, Sychev VN, Levinskikh MA, Alekseev VR, Okuda T, Sugimoto M, Gusev OA, Grigor’ev AI (2009) The biorisk experiment: 13-month exposure of resting forms of organism on the outer side of the Russian segment of the international space station: preliminary results. Doklady Biol Sci 426:267–270
Battista JR (1997) Against all odds: the survival strategies of Deinococcus radiodurans. Annu Rev Microbiol 51:203–224
Bauermeister A, Hahn C, Rettberg P, Reitz G, Moeller R (2012) Roles of DNA repair and membrane integrity in heat resistance of Deinococcus radiodurans. Arch Microbiol 194:959–966
Berger T, Hajek M, Bilsk P, Kőrner C, Vanhavere F, Reitz G (2012) Cosmic radiation exposure of biological test systems during the EXPOSE-E mission. Astrobiology 12:387–392
Blasius M, Hübscher U, Sommer S (2008) Deinococcus radiodurans: what belongs to the survival kit? Crit Rev Biochem Mol Biol 43:221–238
Clark BC (2001) Planetary interchange of bioactive material: probability factors and implications. Orig Life Evol Biosph 31:185–197
Cockell CS (2008) The interplanetary exchange of photosynthesis. Orig Life Evol Biosph 38:87–104
Cox CS (1993) Roles of water molecules in bacteria and viruses. Orig Life Evol Biosph 23:29–36
Daly MJ (2009) A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol 7:237–245
de la Vega UP, Rettberg P, Reitz G (2007) Simulation of the environmental climate conditions on martian surface and its effect on Deinococcus radiodurans. Adv Space Res 11:1672–1677
de Vera J, Boettger U, de la Torre NR, Sánchez FJ, Grunow D, Schimiz N, Lange C, Hübers H, Billi D, Baqué M, Rettberg P, Rabbow E, Reitz G, Berger T, Möller R, Bohmeier M, Horneck G, Westall F, Jänchen J, Fritz J, Meyer C, Onofri S, Selbmann L, Zucconi L, Kozyrovska N, Leya T, Foing B, Demets R, Cockell CS, Bryce C, Wagner D, Serrano P, Edwards HGM, Joshi J, Huwe B, Ehrenfrieund P, Elsaesser A, Ott S, Meessen J, Feyh N, Szewzyk U, Jaumann R, Spohn T (2012) Supporting mars exploration: BIOMEX in Low earth orbit and further astrobiological studies on the moon using Raman and PanCam technology. Planet Space Sci 74:103–110
Dean CJ, Feldschreiber P, Lett JT (1966) Repair of X-ray damage to the deoxyribonucleic acid in Micrococcus radiodurans. Nature 209:49–52
Dewey DL (1969) The survival of Micrococcus radiodurans irradiated at high LET and the effect of acridine orange. Int J Radiat Biol 16:583–592
Dose K, Bieger-Dose A, Kerz O, Gill M (1991) DNA-strand breaks limit survival in extreme dryness. Orig Life Evol Biosph 21:177–187
Dose K, Bieger-Dose A, Dillman R, Gill M, Kerz O, Klein A, Meinert H, Nawroth T, Risi S, Stridde C (1995) ERA-experiment “Space Biochemistry”. Adv Space Res 16:119–129
Fajardo-Cavazos P, Schuerger AC, Nicholson WL (2007) Testing interplanetary transfer of bacteria between Earth and Mars as a result of natural impact phenomena and human spaceflight activities. Acta Astronaut 60:534–540
Gladman BJ, Burns JA, Duncan M, Lee P, Levison HF (1996) The exchange of impact ejecta between terrestrial planets. Science 271:1387–1392
Goodhead DT (1994) Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol 65:7–17
Goossens O, Vanhavere F, Leys N, De Boever P, O’Sullivan D, Zhou D, Spurny F, Yukihara EG, Gaza R, McKeever SWS (2006) Radiation dosimetry for microbial experiment in the international space station using different etched track and luminescent detectors. Radiat Prot Dosim 120:433–437
Green RH, Taylor DM, Gustan EA, Fraser SJ, Olson RL (1971) Survival of microorganisms in a simulated Martian environment. Space Life Sci 3:12–24
Hagen CA, Hawrylewicz EJ, Anderson BT, Cephus MJ (1970) Effect of ultraviolet radiation on the survival of bacteria airborne in simulated Martian dust clouds. Life Sci Space Res 8:53–56
Hellweg CE, Baumstark-Khan C (2007) Getting ready for the manned mission to Mars: the astronauts’ risk from space radiation. Naturwissenschaften 94:517–526
Hirose K, Sugahara H, Matsuno H (2002) Basic performance of VUV exposure system using head-on type Ar2* and Kr2* DBD excimer lamps. J Light Vis Environ 26:35–41
Horneck G (1993) Responses of Bacillus subtilis spores to space environment: results from experiments in space. Orig Life Evol Biosph 23:37–52
Horneck G, Bücker H, Reitz G (1994) Long-term survival of bacterial spores in space. Adv Space Res 14:41–45
Horneck G, Eschweiler U, Reitz G, Wehner J, Willimek R, Strauch K (1995) Biological response to space: results of the experiment “Exobiological Unit” of ERA on EURECA I. Adv Space Res 16:105–118
Horneck G, Rettberg P, Reitz G, Wehner J, Eschweiler U, Strauch K, Panitz C, Starke V, Baumstark-Khan C (2001) Protection of bacterial spores in space, a contribution to the discussion of panspermia. Orig Life Evol Biosph 31:527–547
Horneck G, Miliekowsky C, Melosh HJ, Wilson JW, Cucinotta FA, Gladman B (2002) Viable transfer of microorganisms in the solar system and beyond. In: Horneck G, Baumstark-Khan C (eds) Astrobiology. The quest for the conditions of life. Springer, Berlin, pp 55–76
Horneck G, Moeller R, Cadet J, Douki T, Mancinelli RL, Nicholson WL, Pabitz C, Rabbow E, Rettberg P, Spry A, Stackebrandt E, Vaishampayan P, Venkateswaran KJ (2012) Resistance of bacterial endospores to outer space for planetary protection purposes—experiment PROTECT of the EXPOSE-E mission. Astrobiology 12:445–456
Horneck G, Stöffler D, Ott S, Hornemann U, Cockell CS, Moeller R, Meyer C, de Vera JPP, Fritz J, Schade S, Artemieva NA (2008) Microbial rock inhabitants survive hypervelocity impacts on Mars-like host planets: first phase of lithopanspermia experimentally tested. Astrobiology 8(1):17–44
Horneck G, Klaus DM, Mancinelli RL (2010) Space microbiology. Microbiol Mol Biol Rev 74:121–156
Kobayashi Y, Shimizu T, Tanaka A, Kikuchi M, Taucher-Scholz G, Watanabe H (1995) RBE/LET effects of heavy ions on inactivation in dry cells of Deinococcus radiodurans. JAERI Rev 95-019 pp 44-46
Lean J (1991) Variations in the Sun’s radiative output. Rev Geophys 29:505–535
Mattimore J, Battista JR (1996) Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol 178:633–637
Mennigmann HD (1989) Exobiology: results of spaceflight missions. Adv Space Res 9:3–12
Mileikowsky C, Cucinotta FA, Wilson JW, Gladman B, Horneck G, Lindegren L, Melosh J, Rickman H, Valtonen M, Zheng JQ (2000) Natural transfer of viable microbes in space: I. From Mars to Earth and Earth to Mars. Icarus 145:391–427
Narumi I (2003) Unlocking radiation resistance mechanisms: still along way to go. Trends Microbiol 11:422–425
Nicholson WL (2009) Ancient micronauts: interplanetary transport of microbes by cosmic impacts. Trends Microbiol 17:243–250
Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P (2000) Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64:548–572
Nicholson WL, Schuerger AC, Setlow P (2005) The solar UV environment and bacterial spore UV resistance: considerations for Earth-to-Mars transport by natural processes and human spaceflight. Mutat Res 571:249–264
Olsson-Francis K, Cockell CS (2010) Experimental methods for studying microbial survival in extraterrestrial environments. J Microbiol Methods 80:1–13
Onofri S, de la Torre R, de Vera JP, Ott S, Zucconi L, Selbmann L, Scalzi G, Venkateswaran KJ, Rabbow E, Sánchez Iñigo FJ, Horneck G (2012) Survival of rock-colonizing organisms after 1.5 years in outer space. Astrobiology 12:508–516
Osman S, Peeters Z, La Duc MT, Mancinelli R, Ehrenfreund P, Venkateswaran K (2008) Effect of shadowing on survival of bacteria under conditions simulating the Martian atmosphere and UV radiation. Appl Environ Microbiol 74:959–970
Paulino-Lima IG, Pilling S, Janot-Pacheco E, Naves de Brito A, Ribeiro Gonçalves Barbosa JA, Costa Leitão A, de Alencar Santos Lage C (2010) Laboratory simulation of interplanetary ultraviolet radiation (broad spectrum) and its effects on Deinococcus radiodurans. Planet Space Sci 58:1180–1187
Rabbow E, Rettberg P, Barczyk S, Bohmeier M, Parpart A, Panitz C, Horneck G, von Heise-Rotenburg R, Hoppenbrouwers T, Willnecker R, Baglioni P, Demets R, Dettmann J, Reitz G (2012) EXPOSE-E: an ESA astrobiology mission 1.5 years in space. Astrobiology 12:374–386
Saffary R, Nandakumar R, Spencer D, Robb FT, Davila JM, Swartz M, Ofman L, Thomas RJ, DiRuggiero J (2002) Microbial survival of space vacuum and extreme ultraviolet irradiation: strain isolation and analysis during a rocket flight. FEMS Microbiol Lett 215:163–168
Silverman GJ, Davis SN, Keller WH (1964) Exposure of microorganisms to simulated extraterrestrial space ecology. Life Sci Space Res 2:372–384
Slade D, Radman M (2011) Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev 75:131–191
Takahashi Y, Nakagawa T, Hashimoto H, Shibata S (2011) Survivability of moss and fungal spores in tests simulating conditions of the ISS outer wall. Biol Sci Space 25:83–92
Vanhaverea F, Genicota JL, O’Sullivanb D, Zhouc D, Spurnýd F, Jadrníčkovád I, Sawakuchie GO, Yukihara EG (2008) DOsimetry of BIological EXperiments in SPace (DOBIES) with luminescence (OSL and TL) and track etch detectors. Radiat Meas 43:694–697
Wilson JW, Cucinotta FA, Shinn JL, Simonsen LC, Dubey RR, Jordan WR, Jones TD, Chang CK, Kim MY (1999) Shielding from solar particle event exposures in deep space. Radiat Meas 30:361–382
Yamagishi A, Yano H, Okudaira K, Kobayashi K, Yokobori S, Tabata M, Kawai H, Yamashita M, Hashimoto H, Naraoka H, Mita H (2008) TANPOPO: astrobiology exposure and micrometeoroid capture experiments. Int Symp Space Tech Sci (ISTS) Web Paper Archives 2008-k-05
Yang Y, Itahashi S, Yokobpri S, Yamagishi A (2008) UV-resistant bacteria isolated from upper troposphere and lower stratosphere. Biol Sci Space 22:18–25
Yang Y, Itoh T, Yokobori S, Itahashi S, Shimada H, Satoh K, Ohba H, Narumi I, Yamagishi A (2009a) Deinococcus aerius sp. nov., isolated from the high atmosphere. Int J Syst Evol Microbiol 59:1862–1866
Yang Y, Yokobori S, Yamagishi A (2009b) Bacterial survival in response to desiccation and high humidity at above zero and subzero temperatures. Adv Space Res 43:1285–1290
Yang Y, Yokobori S, Yamagishi A (2009c) Assessing panspermia hypothesis by microorganisms collected from the high altitude atmosphere. Biol Sci Space 23:151–163
Yang Y, Itoh T, Yokobori S, Shimada H, Itahashi S, Satoh K, Ohba H, Narumi I, Yamagishi A (2010) Deinococcus aetherius sp. nov., isolated from the stratosphere. Int J Syst Evol Microbiol 60:776–779
Zagorski ZP (2007) Question2: relation of panspermia-hypothesis to astrobiology. Orig Life Evol Biosph 37:351–355
Zimmermann H, Schäfer M, Schmitz C, Bücker H (1994) Effects of heavy ions on inactivation and DNA double strand breaks in Deinococcus radiodurans R1. Adv Space Res 14:213–216
Acknowledgments
We thank the HIMAC operators for their excellent technical assistance during the heavy-ion irradiation experiments. We also thank Dr. Kosuke Kurosawa for his valuable comments on the methods and physical laws that were used for solving the equations. We also thank all members of the Tanpopo mission for encouraging us on this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawaguchi, Y., Yang, Y., Kawashiri, N. et al. The Possible Interplanetary Transfer of Microbes: Assessing the Viability of Deinococcus spp. Under the ISS Environmental Conditions for Performing Exposure Experiments of Microbes in the Tanpopo Mission. Orig Life Evol Biosph 43, 411–428 (2013). https://doi.org/10.1007/s11084-013-9346-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-013-9346-1