Summary
We performed this study in order to assess the clinical potential of 13N-ammonia PET in patients with cerebral astrocytoma.
Methods
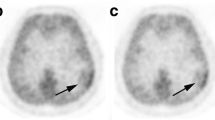
Dynamic 13N-ammonia PET was performed in 25 patients with suspected cerebral gliomas or recurrent cerebral astrocytomas (19 male and 6 female patients; age range 18–64 years) detected by MRI. The histopathological diagnoses were made for all cases either by biopsy or craniotomy, except for one patient with brain infarction and one patient with brain radiation necrosis confirmed by repeated MRI imaging. PET images were visually inspected, and the tumor-to-white matter count (T/W) ratios and the perfusion index (PI) of the tumors were determined.
Results
Six out of nine cases of low-grade gliomas were detected with 13N-ammonia PET, and three non-astrocytoma low-grade gliomas were not detected with 13N-ammonia PET. All 11 high-grade astrocytomas exhibited markedly increased uptake of 13N-ammonia. The five non-neoplastic lesions exhibited low uptake, low T/W ratios and low PI. The significant differences were observed between high-grade and low-grade gliomas with respect to both the T/W ratios and PI (T/W ratios: 5.92±2.27, n=11 vs. 1.66±0.61, n=9, P<0.01; PI: 5.22±1.67, n=11 vs. 1.60±0.54, n=9, P<0.01). There were the significant differences between the T/W ratios and PI in low-grade astrocytomas and that in non-neoplastic lesions (T/W ratios: 2.00±0.42, n=6 vs. 0.97±0.11, n=5, P<0.01; PI: 1.89±0.37, n=6 vs. 0.99±0.03, n=5, P<0.01).
Conclusions
There is a substantial uptake of 13N-ammonia in cerebral astrocytomas. 13N-ammonia PET may enable differentiation between low- and high-grade astrocytomas, and has the potential to enable differentiation between low-grade astrocytomas and non-neoplastic lesions.
Similar content being viewed by others
References
Ricci PE, (1999). Imaging of adult brain tumors Neuroimag Clin N Am 9:651–669
Chung JK, Kim YK, Kim SK, et al, (2002). Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET Eur J Nucl Med 29:176–182
Roelcke U, Radu EW, von Ammon K, et al, (1995) Alteration of blood–brain barrier in human brain tumors: comparison of [18F]fluorodeoxyglucose, [11C]methionine and rubidium-82 using PET J Neurol Sci.132: 20–27
Ogawa T, Shishido F, Kanno I, et al, (1993). Cerebral glioma: evaluation with methionine PET Radiology 186: 45–53
Ohtani T, Kurihara H, Ishiuch S, et al, (2001). Brain tumour imaging with 11C-choline: comparison with FDG PET and gadolinium-enhanced MR imaging Eur J Nucl Med 28: 1664–1679
Kleihues P, Cavenee W, (2000). Pathology and genetics of tumours of the nervous system. International Agency for Research on Cancer; Lyon, pp.107–109
Hoop B, Hnatowich DJ, Brownell GL, et al, (1976). Techniques for positron scintigraphy of the brain J Nucl Med 17: 473–479
Schelstraete K, Simons M, Deman J, et al, (1982) Uptake of 13N-ammonia by human tumours as studied by positron emission tomography Br J Radiol 55: 797–804
Benard F, Romsa J, Hustinx R, (2003). Imaging gliomas with positron emission tomography and single-photon emission computed tomography Semin Nucl Med 33: 148–162
Schelbert HR, Phelps ME, Huang SC, et al, (1981). N-13 ammonia as an indicator of myocardial blood flow Circulation 63: 1259–1272
Phelps ME, Hoffman EJ, Coleman RE, et al, (1976). Tomographic images of blood pool and perfusion in brain and heart J Nucl Med 17: 603–612
Phelps ME, Huang SC, Hoffman EJ, et al, (1981). Cerebral extraction of N-13 ammonia: its dependence on cerebral blood flow and capillary permeability-surface area product Stroke 12: 607–619
Bergmann SR, Hack S, Tewson T, et al, (1980). The dependence of accumulation of 13NH3 by myocardium on metabolic factors and its implications for quantitative assessment of perfusion Circulation 61: 34–43
Cooper AJL, McDonald JM, Gelbard AS, et al, (1979). The metabolic fate of 13N-labeled ammonia in rat brain J Biol Chem 254: 4982–4992
Pilkington GJ, Lantos PL, (1982). The role of glutamine synthetase in the diagnosis of cerebral tumours Neuropathol Appl Neurobiol 8: 227–236
Akimoto J. (1993). Immunohistochemical study of glutamine synthetase expression in normal human brain and intracranial tumors No To Shinkei 45: 362–368
McCormick D, McQuaid S, McCusker, et al, (1990). A study of glutamine synthetase in normal human brain and intracranial tumours Neuropathol Appl Neurobiol 16: 205–211
Mineura K, Sasajima T, Kowada M, et al, (1994). Perfusion and metabolism in predicting the survival of patients with cerebral gliomas Cancer 73: 2386–2394
Pruel MC, Caramanos Z, Collins DL, et al, (1996). Accurate noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy Nature Med 2: 323–325
Somorjai RL, Dolenko B, Nikulin AK, et al, (1996). Classification of H MR spectra of human brain neoplasms: the influence of preprocessing and computerized consensus diagnosis classification accuracy J Magn Reson Imaging 6: 437–444
Taylor JS, Langston JW, Peddick WE, et al, (1996). Clinical value of proton magnetic resonance spectroscopy for differentiating recurrent or residual brain tumor from delayed cerebral necrosis Int J Radiat Oncol Biol Phys 36: 1251–1261
Negendank WG, Sauter R, Brown TR, et al, (1996). Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study J Neurosurg 84: 449–458
Acknowledgement
The authors are highly grateful to the anonymous referees for their significant and constructive critiques and suggestions, which improve the paper very much. This work was partially supported by grant 031652 from the Nature Sciences Foundation of Guangdong Province, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiangsong, Z., Changhong, L., Weian, C. et al. PET Imaging of cerebral astrocytoma with 13N-ammonia. J Neurooncol 78, 145–151 (2006). https://doi.org/10.1007/s11060-005-9069-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-005-9069-x