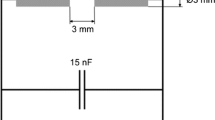
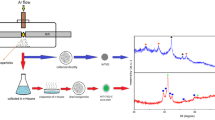
Abstract
Short spark discharges (2 μs) were successfully applied to generate mixed particles a few nanometres in diameter by fast quenching. Alloyed Cr–Co electrodes were applied to demonstrate this. Further it was shown that if the anode and the cathode are different materials, the discharge process mixes the vapour of both materials, forming mixed nanoparticles. Electron microscopy (TEM, SEM), energy dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD) analyses were performed on the collected particles to study their size, morphology, composition and structure. The average compositions of the particles were measured by inductively coupled plasma (ICP). In addition, online measurements of the particle size distribution by mobility analysis were carried out. In the case of alloyed electrodes (Cr–Co), the relative concentration of the elements in the nanoparticulate sample was consistent with the electrode composition. When using electrodes of different metals (Au–Pd and Ag–Pd) the individual nanoparticles showed a range of mixing ratios. No surface segregation was observed in these mixed noble metal particles. Crystalline nanoparticulate mixed phases were found in all cases.











Similar content being viewed by others
References
Barret CS et al (1996) The structure of the metals. McGraw-Hill, New York, p 372
Cundall CM, Craggs JD (1955) Electrode vapor jets in spark discharges. Spectrochim Acta 7:149–164. doi:10.1016/0371-1951(55)80057-4
Devarajan S, Bera P, Sampath S (2005) Bimetallic nanoparticles: a single step synthesis, stabilization, and characterization of Au–Ag, Au–Pd, and Au–Pt in sol–gel derived silicates. J Colloid Interface Sci 290:117–129. doi:10.1016/j.jcis.2005.04.034
Fernández AL, de Pablo L (2002) Formation and the colour development in cobalt spinel pigments. Pigment Resin Technol 31(6):350–356. doi:10.1108/03699420210449043
Gale WF, Totemeier TC (2004) Smithells metals reference book. ASM International, The Materials Information Society, Materials Park
Hansen PM (1958) Constitution of binary alloys. McGraw-Hill, New York
Helsper C, Molter W (1993) Investigation of a new aerosol generator for the production of carbon aggregate particles. Atmos Environ 27A(8):1271–1275
Hinds WC (1999) Aerosol technology, properties, behaviors, and measurements of airborne particles. Wiley, New York
Hirakawa K, Toshima N (2003) Ag/Rh bimetallic nanoparticles formed by self-assembly from Ag and Rh monometallic nanoparticles in solution. Chem Lett 32(1):78–79. doi:10.1246/cl.2003.78
Hume-Rothery W, Raynor GV (1954) The structure of metals and alloys. The Institute of Metals, London
Jenkins NT, Eagar TW (2003) Submicron particle chemistry: vapor condensation analogous to liquid solidification. JOM J Miner Met Mater Soc 55(6):44–47
Kahng S-J, Choi YJ, Park J-Y, Kuka Y (1999) Phase separation in a two-dimensional Co–Cr alloy. Appl Phys Lett 74(8):1087–1089. doi:10.1063/1.123490
Kim J-T, Chang J-S (2005) Generation of metal oxide aerosol particles by a pulsed spark discharge technique. J Electrost 63:911–916. doi:10.1016/j.elstat.2005.03.066
Kim M-J, Na H-J, Lee KC, Yoo EA, Lee M (2003) Preparation and characterization of Au–Ag and Au–Cu alloy nanoparticles in chloroform. J Mater Chem 13:1789–1792. doi:10.1039/b304006m
Kubaschewski O, Alcock CB (1979) Metallurgical thermo-chemistry, international series on materials science and technology, vol 24. Pergamon International Library, Oxford
Liu HB, Pal U, Medina A, Maldonado C, Ascencio JA (2005) Structural incoherency and structure reversal in bimetallic Au–Pd nanoclusters. Phys Rev B71:075403 1–075403 6
Mäkelä JM, Aalto P, Gorbunov BZ, Korhonen P (1992) Size distributions from aerosol spark generator. J Aerosol Sci 23(Suppl 1):S233–S236. doi:10.1016/0021-8502(92)90392-9
Menon M, Khanra BC (2001) Alloying behaviour in Cu–Pd nanostructures. Physica B 304:181–185. doi:10.1016/S0921-4526(01)00340-4
Powell A, Van Den Avyle J, Damkroger B, Szekely J, Pal U (1997) Analysis of multicomponent evaporation in electron beam melting and refining of titanium alloy. Metall Mater Trans 28B(6):1227–1239
Predel B, Madelung O (1998) Phase equilibria, crystallographic and thermodynamic data of binary alloys. Springer, Berlin
Regen MR, Banerjee IA (2006) Preparation of Au–Pd bimetallic nanoparticles in porous germania nanoparticles: a study of their morphology and catalytic activity. Scr Mater 54:909–914. doi:10.1016/j.scriptamat.2005.10.068
Rouquerol F, Rouquerol J, Kenneth SW (1999) Adsorption by powders and porous solids: principles, methodology and applications. Academic Press, San Diego
Schwyn S, Garwin E, Schmidt-Ott A (1988) Aerosol generation by spark discharge. J Aerosol Sci 19(5):639–642. doi:10.1016/0021-8502(88)90215-7
Tabrizi NS, Ullmann M, Vons VA, Lafont U, Schmidt-Ott A (2008) Generation of nanoparticles by spark discharge. J Nanopart Res. doi:10.1007/s11051-008-9407-y
Wang K-W, Chung S-R, Perng T-P (2006) Surface segregation and homogenization of Pd70Ag30 alloy nanoparticles. J Alloy Compd 422:223–226. doi:10.1016/j.jallcom.2005.12.009
Waseda Y, Muramatsu A (2004) Morphology control of materials and nanoparticles. Springer, Berlin
Yang C-C, Wan C-C, Wang Y-Y (2004) Synthesis of Ag/Pd nanoparticles via reactive micelles as templates and its application to electroless copper deposition. J Colloid Interface Sci 279:433–439. doi:10.1016/j.jcis.2004.06.098
Yang Y, Saoud KM, Abdelsayed V, Glaspell G, Deevi S, El-Shall MS (2006) Vapor phase synthesis of supported Pd, Au, and unsupported bimetallic nanoparticles catalysts for CO oxidation. Catal Commun 7:281–284
Acknowledgments
The authors would like to express their gratitude to Patricia Kooyman for her contribution to TEM analysis. The Project is partially funded by the Delft Center of Sustainable Energy (DISE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabrizi, N.S., Xu, Q., van der Pers, N.M. et al. Synthesis of mixed metallic nanoparticles by spark discharge. J Nanopart Res 11, 1209–1218 (2009). https://doi.org/10.1007/s11051-008-9568-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-008-9568-8