Abstract
Chronic, multi-factorial conditions caused by a complex interaction between genetic and environmental risk factors frequently share common disease mechanisms, as evidenced by an overlap between genetic risk factors for cardiovascular disease (CVD) and Alzheimer’s disease (AD). Single nucleotide polymorphisms (SNPs) in several genes including ApoE, MTHFR, HFE and FTO are known to increase the risk of both conditions. The E4 allele of the ApoE polymorphism is the most extensively studied risk factor for AD and increases the risk of coronary heart disease by approximately 40 %. It furthermore displays differential therapeutic responses with use of cholesterol-lowering statins and acetylcholinesterase inhibitors, which may also be due to variation in the CYP2D6 gene in some patients. Disease expression may be triggered by gene-environment interaction causing conversion of minor metabolic abnormalities into major brain disease due to cumulative risk. A growing body of evidence supports the assessment and treatment of CVD risk factors in midlife as a preventable cause of cognitive decline, morbidity and mortality in old age. In this review, the concept of pathology supported genetic testing (PSGT) for CVD is described in this context. PSGT combines DNA testing with biochemical measurements to determine gene expression and to monitor response to treatment. The aim is to diagnose treatable disease subtypes of complex disorders, facilitate prevention of cumulative risk and formulate intervention strategies guided from the genetic background. CVD provides a model to address the lifestyle link in most chronic diseases with a genetic component. Similar preventative measures would apply for optimisation of heart and brain health.
Similar content being viewed by others
Introduction
The rapidly aging population drives an increase in the incidence and prevalence of cardiovascular disease (CVD), which in turn contributes to an explosion of vascular dementia (VaD) and Alzheimer’s disease (AD). Many vascular risk factors such as atherosclerosis, stroke and cardiac disease could result in cerebrovascular dysfunction and trigger AD pathology (Rocchi et al. 2009). Many patients with VaD have clinical features of AD coupled with vascular risk factors and associated strokes. The overlap between AD and cerebrovascular disease therefore produces a disorder that may be amenable to therapeutic approaches based on either mechanism (Kotze et al. 2006). Research efforts are increasingly focused on elucidation of the underlying cause of AD, which has resulted in significant progress in understanding the wide range of both genetic and environmental risk factors that converge into the development and progression of this common neurodegenerative disease.
Prospective follow-up studies performed in nearly 10 000 Californians over a period of 27 years have shown that the same risk factors that lead to the development of CVD in middle age also significantly increase the risk of dementia in old age (Whitmer et al. 2005a,b). Overweight individuals had a 35 % increased risk, while obesity was associated with a 74 % increased risk of dementia compared with normal-weight individuals. High serum cholesterol levels, hypertension, diabetes and smoking between the ages of 40–44 years were associated with a 20–40 % increased risk of dementia in old age. Treatment of hypertension was shown to reduce the risk of dementia by 50 % (Poon 2008) and represents one of several CVD risk factors that can be targeted to protect against cognitive decline. Matsuzaki et al. (2011) investigated whether abnormal lipid metabolism was associated with AD pathology (neuritic plaques and neurofibrillary tangles), and found higher lipid measurements, including cholesterol and triglycerides, in subjects with neuritic plaques. However, there was no relationship of lipid profile parameters with neurofibrillary tangles.
Metabolism and the brain
There is a close link between metabolic processes and brain function. Excessive intake of dietary carbohydrates and particularly high fructose corn syrup, may lead to oxidative damage and ultimately apoptosis of brain cells (Seneff et al. 2011). These findings are in accordance with loss of lipid asymmetry and consequent neurotoxicity as demonstrated in the AD brain using a mouse model (Bader Lange et al. 2010). Phosphatidyldserine asymmetry was significantly altered in an age-dependent manner as a result of oxidative stress and/or apoptosis. The same disease mechanism may apply to other neurodegenerative diseases that share many properties with AD.
In an effort to define dietary patterns that promote cognitive health, Bowman et al. (2012) examined the cross-sectional relationships between nutrient status and psychometric and imaging indices of brain health in 104 dementia-free elders. Distinct nutrient biomarker patterns analysed in plasma were shown to account for a significant degree of variance in both cognitive function and brain volume. More favourable cognitive and MRI measures were significantly associated with two nutrient biomarker patterns: one high in plasma vitamins B (B1, B2, B6, folate and B12), C, D and E, and another high in plasma marine omega-3 fatty acids. A high trans fat pattern was consistently associated with worse cognitive performance and less total cerebral brain volume. Trans fats may replace docosahexaenoic acid (DHA) in neuronal membranes and high intake increases the risk of CVD, systemic inflammation, and endothelial dysfunction that could explain the deleterious effect on cognitive decline (Lopez-Garcia et al. 2005; Schaefer et al. 2006; Mozaffarian et al. 2006). Depression attenuated the relationship between the omega-3 fatty acids and white matter hyperintensity volume (Bowman et al. 2012) and should be addressed as part of a comprehensive CVD and AD risk reduction strategy.
A diet protective against CVD and AD includes reduced intake of animal products, especially red and organ meats and high-fat dairy and avoidance of trans fats, and high intake of fish, fruit, dark and green leafy vegetables and cruciferous vegetables (Morris et al. 2004; Scarmeas et al. 2006; Barberger-Gateau et al. 2007; Gu et al. 2010). However, large clinical trials failed to show the benefit of vitamin E, B vitamins or DHA (Aisen et al. 2008; Bowman et al. 2012) despite several previous studies in favour of the important role of certain nutrients in the prevention of AD. Given the differences in dietary patterns between populations, and the interactive nature of nutrient action and metabolism, contradictory findings are not surprising. Individual genetic differences in the absorption and utilisation of nutrients in the diet could also affect the risk of disease development and progression. This underscores the rationale for an integrative genomic healthcare approach that collectively incorporates the influence of multiple genetic and environmental risk factors in relation to protective nutrients in a new integrative medical model for optimising vascular and brain health.
In this review the genetic link between CVD and AD is discussed in relation to single nucleotide polymorphisms (SNPs) in the Apo E, MTHFR, HFE and FTO genes implicated in both conditions. These genes are involved in the metabolism of fat and cholesterol, folate and homocysteine, and iron dysregulation in the liver that is closely related to glucose secretion. We provide the rationale for use of a pathology supported genetic testing (PSGT) approach towards healthy aging. The aim is to 1) diagnose treatable genetic subtypes of complex diseases as early as possible, 2) facilitate the prevention of cumulative risk and 3) formulate intervention strategies tailored to the needs of the individual. PSGT is applied with use of the Gknowmix Database (https//:www.gknowmix.com) that matches known genetic and environmental risk factors identified in patients with their medical history and biochemical measurements, allowing for gene expression and response to treatment to be determined and monitored as part of routine clinical care.
Cholesterol metabolism
Although serum and brain cholesterol are two separate pools (Bjorkhem 2006) high serum total cholesterol in midlife is associated with an increased risk of both AD and VaD (Solomon et al. 2009). Based on the finding that even moderately elevated cholesterol increases the risk of dementia, these authors recommended early intervention before underlying disease(s) or symptoms appear.
Prior to the development and implementation of a treatment plan in patients with high cholesterol levels, the extent to which genetic risk factors may play a role needs to be determined. An accurate diagnosis is a prerequisite for optimal treatment and the appropriateness of genetic testing should only be considered after careful documentation of other potential risk factors. These include a family history of hypercholesterolaemia and CVD, personal medical history and current health status, as well as environmental risk factors such as smoking and body mass index (BMI) known to influence cholesterol levels. Distinction of patients with familial hypercholesterolaemia (FH) from those with less severe forms of dyslipidaemia is very important in the South African population, where the prevalence of this lipid disorder is increased 5–10 times compared to most other populations due to a founder effect (Kotze et al. 2003). While nutrition and lifestyle modifications are sufficient to normalise cholesterol levels in most hypercholesterolaemics, FH patients additionally require long-term drug treatment to reduce the risk of premature heart attacks.
Lessons learned from extensive study of FH in the genetically distinct populations of South Africa have led to a modified approach to risk management of CVD, which culminated in the PSGT approach. Nearly 20 years ago we demonstrated for the first time that the same FH mutation could be associated with variable clinical expression, ranging from occurrence of a myocardial infarction at an early age (<50 years) to good health into advanced age (>80 years) in the same family, depending on gene-gene and gene-environment interaction (Kotze et al. 1993a). The type or severity of the gene defect furthermore affects serum cholesterol levels differentially, although not sufficiently to explain clinical variability in coronary events (Kotze et al. 1993b). Based on the knowledge that high-risk environments and modifier genes affecting disease expression in monogenic conditions such as FH would also increase the risk of CVD in the general population, a comprehensive CVD multi-gene test performed in conjunction with a medical and lifestyle assessment was developed (Kotze et al. 2003; Kotze and Thiart 2003). This CVD testing approach summarised in Table 1 can be applied in patients at risk of both CVD and AD (Kotze et al. 2006). The mutations or functional polymorphisms included in the CVD assay was based on their phenotypic effect, allele frequencies in the local population and availability of appropriate intervention or treatment options. The genotypes being tested (i) affect the function or level (expression) of the gene products, (ii) affect biological processes involved in CVD and related disorders, and (iii) have apparent metabolic/clinical implications, either alone or in combination with other genetic or environmental risk factors.
FH test
The first test option in Table 1 includes eight mutations in the low-density lipoprotein receptor (LDLR) gene (D154N, Del197, D200G, D206E, C356Y, G361V, V408M, P664L) (Kotze et al. 2003). These mutations account for FH in the majority of affected patients in the high-risk Afrikaner, Indian and Ashkenazi-Jewish populations of South Africa. Less than 10 % of FH patients in South Africa have been correctly diagnosed, despite extensive awareness and publicising of hypercholesterolaemia as a CVD risk factor (Marais et al. 2004). Vergotine et al. (2001) have demonstrated that determination of total cholesterol levels in FH families with known mutations fails to provide the correct diagnosis in nearly 30 % of individuals when the 95th percentile for age and gender is used, and in 12 % of cases when the 80th percentile is used. Of the 60–70 % of South Africans with high cholesterol levels, 5–10 % will have FH. This distinction is very important for treatment considerations to identify those at highest risk, as male FH patients have a more than 50 % risk of coronary heart disease by age 50 years and in females the risk is approximately 30 % by age 60. In the high-risk South African population the average age of death is 45 years in men with FH (Marais et al. 2004).
CVD multi-gene test
The second test option, performed in conjunction with a medical and lifestyle assessment, includes multiple mutations of relatively low expression in different CVD-related genes. In addition to the dyslipidaemia-related mutations included in the CVD multi-gene assay, genetic variations involved in folate metabolism (methylation), haemostasis (blood clotting) and iron overload are also assessed (Kotze et al. 2003; Kotze and Thiart 2003). These include functional SNPs in the ApoE, (rs429358 and rs7412), MTHFR (rs1801133 and rs1801131), F2 (rs1799963), FV (rs6025) and HFE (rs1800562 and rs1799945) genes. The genetic test results are integrated with clinical indicators of CVD risk (Table 1) and lifestyle factors to identify a combination of risk factors that could cause or contribute to disease development, if left untreated.
Since a prospective genotype-phenotype correlation study has not been performed to date to illustrate the direct correlation between different levels of the specific combination of biochemical parameters and the conditions listed in Table 1, continuous monitoring of health outcomes by participating clinicians are encouraged using a combined service and research approach. This strategy is in line with the view of Artinian et al. (2010) who recommended careful examination of the effectiveness of the intervention strategy in routine clinical practice, as opposed to efficacy trails that examine interventions under more structured ideal conditions. The 2020 Goals of the American Heart Association include a new concept of cardiovascular health that incorporates lifestyle risk factors considered key drivers of disease in genetically predisposed individuals. A multi-disciplinary risk reduction approach is necessary since all the risk factors that may lead to complex diseases such as CVD have not yet been elucidated.
The clinical usefulness of this PSGT approach was assessed as part of a nutrition intervention study in South African patients with the metabolic syndrome (MetS) (van Velden et al. 2007). Use of the multi-gene CVD assay in these patients could partly explain differential responses to nutrition intervention because of their genetic background. Genetic testing revealed that two of the twelve MetS patients included in this pilot study had FH, while four individuals tested positive for the cholesterol-raising E4 allele of the apolipoprotein E (ApoE) gene. The ApoE polymorphism occurs in 30–40 % across ethnic groups and is associated with abnormal lipid levels, as also confirmed in the general South African population (Kotze et al. 1993b). The deleterious effects of the ApoE polymorphism are mediated by modifiable environmental risk factors such as smoking, diabetes and obesity, which increase the risk of both CVD and AD (Whitmer et al. 2005a, b). While FH patients require long-term drug treatment with cholesterol-lowering statins, diet and lifestyle modification is the treatment method of choice in hypercholesterolaemics with the Apo E4 allele.
The PSGT approach combines genetic testing with blood biochemistry (pathology) tests to determine gene expression and to monitor the effectiveness of the treatment strategy advised. Identification of a genetic risk factor in the presence of high cholesterol levels would confirm a genetic contribution to the risk profile, and in some patients a treatable genetic subtype such as FH or type III dysbetalipoproteinaemia may be diagnosed after taking all known risk factors documented into account. When a cholesterol-raising genetic risk factor is identified in the presence of normal cholesterol levels it does not mean that the individual has a disease or will develop CVD, but that a genetic predisposition was identified that may in future turn into disease if a high-risk environment is entered. High cholesterol levels or CVD in the absence of any of the genetic risk factors tested for may furthermore point to lifestyle risk factors such as smoking or obesity as the main causative factors, if present. These clinical features may also be caused by the inheritance of other genetic risk factors not included in the initial genetic analysis. Based on the family history and health status of the individual extended mutation analysis may be recommended in some patients, as part of the diagnostic work-up towards development of a pathology supported gene-based risk reduction plan. According to Artinian et al. (2010) a programme of counselling with extended follow-up performed in conjunction with self-monitoring and goal-setting provides the best approach to sustainable lifestyle changes to improve clinical outcome. Our PSGT approach requires regular monitoring (e.g. 6-monthly) of blood biochemistry levels, where appropriate, to assess the effectiveness of the intervention strategy.
In addition to its role in lipid metabolism, variation in the ApoE gene is also associated with inflammation and oxidative stress. This may explain why smoking exacerbates the deleterious effect of the ApoE E4 allele on arterial wall thickening (Humphries et al. 2001). ApoE E4 was shown to be more susceptible to oxidation than the E2 and E3 isoforms and in mice, atherosclerosis could be reversed by dietary vitamin E supplementation (Pratico et al. 1998). To achieve healthy aging, life-long low dietary fat intake appears to be especially important in individuals with the ApoE E4 allele (Petot et al. 2003). When plasma cholesterol levels are raised, individuals with the ApoE E4 allele are most responsive to a low-calorie diet, and less responsive to statin therapy than E2 allele carriers (Gerdes et al. 2000). While moderate alcohol intake appears to have a beneficial effect in relation to CVD risk and cognitive decline it may not be the case in ApoE E4 allele carriers. Anttila et al. (2004) have shown that the risk of dementia increases with increasing alcohol consumption only in those individuals carrying the ApoE E4 allele. A cross-sectional study performed in 685 AD patients from three different ethnic groups confirmed the deleterious effect of heavy smoking (one or more packs per day) and alcohol consumption (more than 2 drinks per day) on cognitive function. Detection of the ApoE E4 allele, a history of heavy smoking or a history of heavy drinking was each associated with a 2–3 years earlier onset of AD. In patients with all three risk factors AD was diagnosed on average 10 years earlier than in those with none of the risk factors (Harwood et al. 2010).
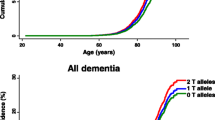
According to Seshadri et al. (1995), the lifetime risk of developing AD is approximately 15 % for persons with no family history and increases to approximately 30 % in carriers with at least one ApoE E4 allele, compared with less than 10 % for those without the E4 allele. In a more recent study it was reported that by the age of 85 years, the lifetime risk of AD without reference to genetic risk factors is approximately 11 % in males and 14 % in females. However, in the presence of the ApoE E4 allele the risk increases to 51 % in males and 60 % in females homozygous for the ApoE E4 allele. In heterozygous carriers the risk was 23 % in males and 30 % in females, consistent with semi-dominant inheritance of a moderately penetrant gene (Genin et al. 2011). Estimates were globally similar and reached the highest lifetime risk in some European populations, of up to 68 % and 35 % for females homozygous or heterozygous for the ApoE 4 allele, respectively. Stratification of the data by age groups demonstrated that the ApoE E4 allele is a risk factor not only for late-onset but for early-onset AD as well.
Folate and homocysteine metabolism
Several studies support a role of one-carbon metabolism in the development of late-onset Alzheimer’s disease. The protective effect of omega-3 fatty acids discussed above may be mediated primarily through the vascular system, while cognitive benefit from a plasma profile high in antioxidants appears to affect the rate of total brain atrophy related to an AD type pathology. This includes reduction of oxidative stress (Bowman et al. 2009; Karuppagounder et al. 2009) and hyperhomocysteinaemia-induced neurotoxicity (Troen et al. 2008). Since homocysteine accumulation contributes significantly to the risk of both CVD and AD (Seshadri et al. 2002; Ravaglia et al. 2005), early detection of genetically increased requirements for folate and other vitamin co-factors are essential for optimal enzyme function and consequently vascular and brain health. Detection of variation in the methylenetetrahydrofolate reductase (MTHFR) gene was shown to be most important in this context and provides one of the best examples of gene-diet interaction (nutrigenetics).
In a study performed by Coppede et al. (2011) the authors demonstrated a significantly increased frequency of the T-allele of the MTHFR 677C>T polymorphism and the G-allele of the MTRR 66A>G polymorphism as well as respective heterozygous and homozygous genoptypes in 378 late-onset AD patients compared with 308 matched controls. These findings correlated significantly with increased mean plasma homocysteine levels and decreased serum folate levels. Detection of an interaction between the studied polymorphisms and biochemical biomarkers underlines the importance of a combination approach. It enables evaluation of the effect of both genetic (e.g. MTHFR mutation) and environmental factors (e.g. folate intake, smoking, alcohol) on accumulation of homocysteine in plasma.
Decreased MTHFR activity due to genetic variation is of special concern in individuals with high alcohol intake since this may lead to impaired folate status due to malabsorption, increased excretion, or abnormal folate metabolism (Halsted et al. 2002). The negative effects of low intake of the methyl-related nutrients with high intakes of alcohol are additive, therefore changes in overall dietary patterns are recommended to ensure the consumption of a protective high methyl diet. This is essential not only to reduce CVD risk but also to preserve cognitive function (Ravaglia et al. 2005).
A meta-analysis performed in 3299 AD patients and 4363 controls has shown that the MTHFR T-allele increases the risk for AD in the general population, particularly in Asian populations (Hua et al. 2011). These findings are in accordance with the meta-analysis performed a year earlier including 19 case-control studies (Zhang et al. 2010). The frequency of the MTHFR T-allele was found to be significantly associated with susceptibility to AD in all subjects. In a subgroup analysis of individuals without the ApoE E4 allele, the association of the MTHFR 677T-allele with AD susceptibility was confirmed in Asians but not in Caucasians. The MTHFR T-allele and mainly the TT genotype, was found to increase the risk of VaD in Asians (Liu et al. 2010a). Population difference may be explained by the fact that genetic influences on homocysteine levels or disease risk would only be detectable in populations with low folate status.
Food fortification and folic acid supplementation represent confounding factors that need to be taken into account in research studies and clinical trials (Holmes et al. 2011). In a study performed by Shmeleva et al. (2003) in North Western Russia where the population has limited access to multivitamins and no food fortification, homozygosity for the T-allele of the MTHFR 677>T polymorphism was detected in all persons with homocysteine levels above 30 μm/l. Amongst carriers with the T allele, thrombotic risk increased 1.9-fold in patients with arterial thrombosis, 1.7-fold for venous thrombosis and 2.5 fold for both conditions. These findings are in accordance with the risk for recurrent venous thrombosis ascribed to the MTHFR genotype by Keijzer et al. (2002). These authors reported a relative risk of between 1.4 and 1.6 in the presence of the T-allele and a combined risk of 18.7 if detected in the presence of the factor V Leiden mutation, the most common genetic risk factor for venous thrombosis. Detection of one or more genetic risk factors causing abnormal blood clotting in patients with venous thrombosis represents another preventable CVD subtype identifiable with the multigene assay referred to in Table 1.
Nutritional requirements differ according to the MTHFR genotype (Moriyama et al. 2002; Herrmann et al. 2003). Individuals with genetic variations in the MTHFR gene may require increased amounts of folate above the recommended daily allowance (RDA) of 400 μg per day, together with adequate amounts of vitamins B6 and B12. Determination of homocysteine levels and genetic testing performed in conjunction with dietary assessment may therefore serve as useful parameters for improvement of a person’s diet. It is not sufficient to perform a biochemical test of plasma homocysteine levels alone, as these levels fluctuate as a consequence of environmental changes (e.g. medication, diet, alcohol intake). A specific DNA test, on the other hand, is performed only once in a lifetime (1) to determine the underlying cause of high homocysteine levels and/or (2) to determine the appropriateness of food supplementation and dosages. As elevated plasma homocysteine is a marker of folate and vitamin B12 deficiency that may lead to many common disorders including CVD and AD, it is important to focus on the prevention of hyperhomocysteinaemia.
Before wide implementation of genetic testing could be promoted in the context of CVD, however, consideration had to be given to the uncertainty of whether the association between elevated serum homocysteine levels and CVD is indeed causal. The meta-analysis performed by Wald et al. (2002) provided strong evidence for causality, because both genetic and prospective studies (that do not share the same potential sources of error) yielded highly significant results. Lowering of homocysteine concentrations by 3 mmol/l by increasing folic acid intake was predicted to reduce the risk of ischaemic heart disease by 16 %, deep vein thrombosis by 25 %, and stroke by 24 % (Wald et al. 2002). In a meta-analysis performed by Klerk et al. (2002), individuals with two copies of the most extensively studied 677C>T mutation (T-allele) were found to have a 16 % higher risk of CHD compared with individuals homozygous for the common allele. The increased risk of stroke associated with the T-allele was furthermore confirmed in a meta-analysis involving 14870 individuals (Cronin et al. 2005). The benefits of folic acid supplementation are explained largely by favourable endothelial function outcomes after lowering homocysteine levels, as assessed by flow-mediated vasodilation or haemostatic markers (Brown and Hu 2001). Genetic testing may identify individuals at risk of homocysteine accumulation before damage occurs to DNA, arteries and the brain.
Vascular risk factors such as high homocysteine levels may modify the neurodegenerative process in patients with mild cognitive impairment (MCI) at increased risk of VaD and AD. The MTHFR 677 TT genotype was shown to promote plasma homocysteine increase which in turn may favour intima-media thickening in patients with cognitive impairment (Gorgone et al. 2009). In a one year follow-up study of 55 patients with mild cognitive impairment and 44 controls matched for age, gender and education, vascular risk factors were found significantly more often in the MCI group, including the ApoE E4 allele (p = 0.018), hyperhomocysteinaemia (p-0.012) and folate deficiency (p = 0.023) (Siuda et al. 2009). However, whether these findings translate into significant changes at the clinical level is uncertain as only age and hypertension influenced the progression from MCI to dementia within one year of this prospective observation. The influence of the ApoE E4 allele on the risk of AD was independent of MTHFR mutation status and homocysteine levels (Styczyńska et al. 2008).
Iron metabolism
The importance of iron metabolism extends beyond the field of nutrition, representing a key factor in pathology, cardiology, oncology, neurological and infectious diseases. Brain iron increases with age and is elevated in AD and several other neurodegenerative diseases, correlating with earlier age of onset in men. In a recent study performed by Bartzokis et al. (2011) it was shown that higher accumulation of iron in vulnerable gray matter regions may represent a risk factor for accelerated cognitive decline. Variation in numerous genes may interact with each other and the diet to determine iron levels in serum and the brain and age of onset of AD (van Rensburg et al. 2000; Sampietro et al. 2001). Bartzokis et al. (2010) reported that highly prevalent genetic variants in iron metabolism genes can influence brain iron levels in men. Identification of the mechanisms underlying brain iron accumulation may lead to novel ways to offer neuroprotection for age-related neurodegenerative diseases.
More than 10 years ago we demonstrated an earlier age of onset in South African patients with AD with both the ApoE E4 allele and the transferrin (TF) C2 polymorphism (Van Rensburg et al. 2000). The significance of TF C2 in AD has subsequently been confirmed in several studies, including the Epistasis Project conducted by Lehmann et al. (2012). The previously reported interaction between the C282Y mutation in the haemochromatosis (HFE) gene and TF C2 in Northern Europeans was confirmed in a comparative study of 1757 patients with AD and 6295 controls. Homozygosity for both the HFE H63D mutation and another variation in the TF gene were also found to be significantly associated with iron loading. Based on these findings, it was concluded that treatment for iron overload may thus be protective in some cases.
The TF C2 variant has an increased allele frequency in AD patients compared to controls in some populations (Van Rensburg et al. 1993; Zambenedetti et al. 2003), but not in others, such as in a French population of the Bordeaux region (Rondeau et al. 2006). Since TF C2 is associated with diseases attributed to oxidative damage, it may be hypothesised that diet may have an effect on the expression of the TF C2 variant. In an environment such as Bordeaux, where a predominantly Mediterranean diet and red wine rich in anti-oxidants are consumed regularly, the deleterious effects of TF C2 variant may be neutralised (Van Rensburg et al. 2010).
The deleterious effects of mutations in the HFE gene are usually ascribed to their role in cellular iron overload. However, in the case of mutation H63D found to be over-represented in AD patients, the mutant protein is associated with prolonged stress of the endoplasmic reticulum and chronically increased neuronal vulnerability as demonstrated in an inducible expression cell model (Liu et al. 2011). While the C282Y mutation is more commonly associated with hereditary haemochromatosis (considered a preventable cause of heart disease and other equally important clinical conditions due to potential organ damage), H63D has received more attention for its role in neurodegenerative diseases. HFE gene variants are considered important in this context due to the association of the HFE H63D mutant protein with iron dyshomeostasis, increased oxidative stress, tau phosphorylation, glutamate release and inflammation at the cellular level (Nandar and Connor 2011).
The importance of assessing genetic risk factors together with relevant blood biochemistry parameters was demonstrated in the study of Giambattistelli et al. (2011). These authors explored the interconnectedness of genetic variation in the HFE (H63D and C282Y) and TF (C2) genes with serum markers of iron status, including iron, ferritin, TF and TF-saturation, as well as liver function (albumin, transaminases) and prothrombin time in 160 AD patients and 79 healthy controls. The results of the liver function tests indicated distress of the liver. TF concentration was lower and ferritin higher in AD patients. Statistical analyses revealed that a one-unit TF serum decrease increases the probability of AD by 80 %, while a one-unit increase of AST/ALT ratio generates a 4-fold probability increase. The HFE H63D mutation was associated with higher levels of iron and lower levels of TF. These results suggest that the presence of HFE mutations and iron abnormalities may increase the probability of developing AD when accompanied by distress of the liver.
In contrast to the above studies providing strong evidence that the HFE H63D mutation is a risk factor for AD when evaluated in conjunction with other relevant biomarkers to assess cumulative effects, a meta-analysis of 22 studies performed in 4365 cases and 8652 controls implicated it as a protective factor (Lin et al. 2011). The previously reported association of HFE C282Y with increased risk of AD could also not be confirmed.
We believe that the only way to overcome the limitations of low-penetrance genetics is to combine DNA tests with relevant pathology or blood biochemistry such as cholesterol, serum iron status, oxidative stress, inflammatory markers and liver function tests as appropriate. For example, biochemical abnormalities associated with low penetrance mutations in the Apo E (cholesterol) and HFE (ferritin, transferrin saturation) genes, respectively, are usually not detectable early in life. As the pathology may develop over a long period of time, preventative measures can be implemented based on this knowledge when a genetic risk factor is identified in the presence of known environmental triggers that could be eliminated or targeted during intervention. Application of the PSGT approach has been discussed previously in the context of iron metabolism (Kotze et al. 2009). Hereditary haemochromatosis (HH) provides an excellent example of a complex disease that is best addressed by the PSGT approach, which requires that (1) genetic testing is performed within a specific pathology/biochemical and clinical profile that also defines the test selection criteria, (2) the patient report contains both the biochemical and genetic test results for clinical application in the context of relevant documented environmental factors, and (3) gene expression and monitoring of response to treatment are assessed through the accompanying pathology and genetic test parameters. This approach has proved valuable to distinguish patients with HH from those with the dysmetabolic or insulin-resistance iron overload disorder frequently observed in patients with non-alcoholic fatty liver disease (NAFLD). NAFLD is the hepatic manifestation of MetS and is considered an independent risk factor for CVD. Ghareeb et al. (2011) have demonstrated that NAFLD may induce insulin resistance and metabolic disorders associated with age-associated neurodegenerative diseases such as AD.
The identification of the spectrum of risk factors underlying iron overload provides a major healthcare opportunity to reduce the burden of dementia, heart disease, cancer, diabetes, arthritis, infertility and many other complications of organ damage in the general population.
Body mass index
Recently, variation in the fat mass and obesity-associated (FTO) gene was identified as the most significant genetic risk factor for susceptibility of polygenic obesity. With more than 300 million obese persons worldwide it is crucial to understand the clinical implications of genetic variation in the FTO gene (Ho et al. 2010). A functional SNP in intron 1 of the FTO gene (rs9939609) (Berulava and Horsthemke 2010) occurs in nearly 50 % of the general population and is associated with an approximately 1.2 kg higher weight, on average, in adults and an approximately 1 cm greater waist circumference (Liu et al. 2010a, b). The effect of the FTO gene to increase the risk of obesity and diabetes may be mediated through epigenetic changes (Almén et al. 2012). Both a high BMI and diabetes linked to the FTO gene are vascular risk factors implicated in the development of VaD and AD.
Much interest is currently focused on the possibility that the FTO gene affects brain function and structure as a high BMI correlates with frontal, temporal and subcortical atrophy (Bertram and Heekeren 2010). FTO is highly expressed in the human brain and primarily affects frontal lobe-dependent cognitive processes (Benedict et al. 2011). This was shown in a study of verbal fluency performance in 355 elderly men who had no clinically apparent cognitive impairment at the age of 82 years. Obese and overweight but not normal weight individuals with the FTO A-allele showed a lower performance on verbal fluency than homozygotes for the T-allele (Benedict et al. 2011).
Generation of 3D maps of regional brain volume revealed a pattern of systematic brain volume deficits in subjects with FTO obesity-associated risk alleles when compared with non-carriers (Ho et al. 2010). The average brain volume difference for the frontal lobes and the occipital lobes were approximately 8 % and 12 %, respectively, with the greatest effects in individuals with high BMI’s. These brain differences were not attributable to the effect of cholesterol, hypertension or the volume of white matter hyperintensities.
In a nine-year prospective follow-up study exploring the impact of FTO on AD and dementia risk in 1003 persons without dementia, individuals with the FTO AA genotype showed a higher risk of cognitive decline after adjustment for age, gender, education, physical activity, BMI, diabetes, CVD and ApoE genotype (Keller et al. 2011). Co-inheritance of both the FTO AA genotype and Apo E4 allele was associated with a significantly increased risk of dementia, and particularly AD. The FTO gene effect acts mostly through interaction with the Apo E4 allele, independently of physical activity, BMI, diabetes and CVD. These results are in accordance with an association between FTO and reduced brain volume reported by Ho et al. (2010).
In a study performed by Moleres et al. (2011) the strong influence of dietary fatty acid distribution on the effect of the rs9939609 polymorphism in the FTO gene on obesity risk was clearly demonstrated from childhood. Genotyping of rs9939609 in 354 Spanish children and adolescents aged 6–18 years (49 % males), showed a statistically significant interaction between the consumption of saturated fatty acids (SFA) (percentage of total energy) in relation to polyunsaturated fatty acid (PUFA) intake and obesity risk linked to the rs9939609 SNP of the FTO gene. Risk allele carriers consuming more than 12.6 % SFA (of total energy) had an increased obesity risk compared with TT carriers. In a similar way, A allele carriers with an intake ratio lower than 0.43 PUFA:SFA presented a higher obesity risk than TT subjects. These findings emphasise the importance of early detection of a genetic predisposition for obesity so that preventative measures can be implemented to prevent cumulative risk that may lead to CVD and/or AD.
Genetic testing for optimised treatment
The above-mentioned information highlights the importance of early identification of CVD risk factors and timely intervention before cognitive decline becomes apparent. While current practice is generally to address symptoms as they occur, several studies point out the importance of treating CVD risk factors as early as midlife (Solomon et al. 2009).
According to the World Health Organisation eight risk factors account for over 75 % of CVD, namely hypertension, high body mass index, high cholesterol, high blood glucose, low fruit and vegetable intake, high alcohol consumption and physical inactivity. In a study performed in more than 800 Caucasians (Szolnoki et al. 2003) it was shown that the presence of the Apo E4 allele worsened the unfavourable effects of hypertension, diabetes mellitus, smoking, or drinking on the incidence of ischaemic stroke. Synergistic effects were also found between the MTHFR 677TT genotype and alcohol consumption or smoking, while detection of a genetic predisposition for venous thrombosis (e.g. factor V Leiden mutation) in combination with hypertension and diabetes mellitus increased the risk of ischaemic stroke. A study performed by Volzke et al. (2005) confirmed the increased risk of atherosclerosis with rising serum LDL-cholesterol concentrations in carriers of the factor V Leiden mutation. In a study performed in 12 239 women between the ages of 51 to 69 years, Roest et al. (1999) demonstrated that inherited variation in iron metabolism (HFE gene) is involved in cardiovascular death in postmenopausal women, especially in women already carrying classic risk factors such as hypertension and smoking (Roest et al. 1999). These findings confirm the importance of early detection of a genetic contribution to clinical risk factors for disease prevention.
Therapeutic responses may also be genotype specific and this could contribute to the apparent inability of presently available drugs to alter the course of AD for some patients. Approximately 15 % of AD cases with adverse response to treatment are associated with a defective CYP2D6 gene which can be assessed genetically (Cacabelos 2003). Pharmacogenetic response may be influenced not only by this gene involved in drug metabolism, but also genes involved in the pathogenesis of both CVD and AD. Patients with the ApoE E4 allele appear to be the worst responders to both cholesterol-lowering statin treatment and various drugs prescribed to AD patients (Cacabelos 2008). This author suggested that the presence of the ApoE-44 genotype could convert CYP2D6 extensive metabolisers into poor metabolisers. If confirmed in future studies, gene-gene interaction between the Apo E and CYP2D6 genes may have important clinical implications for treatment of a wide range of chronic disorders.
The key to disease prevention therefore lies in a better understanding of gene-environment interactions underlying CVD and AD and effective intervention based on this knowledge (Fig. 1) (Kotze et al 2006). Genetic testing enables the dissection of complex conditions into treatable subtypes; for example to distinguish between FH patients requiring long-term drug treatment and those with the ApoE E4 allele who respond well to specific dietary and lifestyle changes without the use of medication.
Co-inheritance of multiple genetic risk factors in the presence of environmental triggers has a significant impact on the development of clinical conditions associated with the development of cardiovascular disease (CVD) and/or Alzheimer’s disease (AD). Environmental factors that may be either harmful or beneficial (right column) in the context of their direct effect on genetic factors underlying the high-risk clinical conditions highlighted in the circles on the outside, can potentially be manipulated to prevent the conversion of genetic risk factors into disease (Reproduced with permission from Kotze et al. 2006)
Conclusions
Genetic testing of CVD risk factors has the potential to translate into improved health outcomes in patients with cognitive impairment and their at-risk family members. Based on the knowledge that assessment and treatment of CVD risk factors in middle age may reduce the risk of heart disease and dementia in old age (Kotze et al. 2006), a comprehensive PSGT approach was developed. Since the deleterious effects of several genetic risk factors shared between CVD and AD are mediated through diet and lifestyle factors, identification of a genetic predisposition highlights the importance of smoking cessation where relevant and a healthy diet with regular intake of foods that offer neuroprotection. In contrast to severe CVD subtypes such as FH that requires aggressive treatment, simple lifestyle changes can delay the onset and severity of dementia (Potocnik et al. 2005).
Contrary to previous belief, the identification of AD genetic risk factors that may be triggered by environmental factors does not result in increased worrying and depression (Romero et al. 2005). Genetic testing for multiple CVD risk factors in conjunction with nutrition and cognitive assessment may empower patients to take the necessary steps to improve their health. The remaining gaps in our knowledge Hudson et al. (2012) necessitate a transdisciplinary PSGT approach linked to ongoing health outcome studies. Educational campaigns that highlight the link between CVD risk factors and AD may help reduce the impact of both conditions.
References
Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, Bottiglieri T, Jin S, Stokes KT, Thomas RG, Thal LJ, Alzheimer Disease Cooperative Study (2008) High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 300:1774–1783
Almén MS, Jacobsson JA, Moschonis G, Benedict C, Chrousos GP, Fredriksson R, Schiöth HB (2012) Genome wide analysis reveals association of a FTO gene variant with epigenetic changes. Genomics Jan 2 [Epub ahead of print]
Anttila T, Helkala EL, Viitanen M, Kåreholt I, Fratiglioni L, Winblad B, Soininen H, Tuomilehto J, Nissinen A, Kivipelto M (2004) Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. Br Med J 329:539
Artinian NT, Fletcher GF, Mozaffarian D, Kris-Etherton P, Van Horn L, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS, Meininger JC, Banks J, Stuart-Shor EM, Fletcher BJ, Miller TD, Hughes S, Braun LT, Kopin LA, Berra K, Hayman LL, Ewing LJ, Ades PA, Durstine JL, Houston-Miller N, Burke LE, American Heart Association Prevention Committee of the Council on Cardiovascular Nursing (2010) Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation 122:406–441
Bader Lange ML, St Clair D, Markesbery WR, Studzinski CM, Murphy MP, Butterfield DA (2010) Age-related loss of phospholipid asymmetry in APP(NLh)/APP(NLh) x PS-1(p264L0/PS-1(P264L) human double mutant knock-in mice: relevance to Alzheimer’s disease. Neurobiol Dis 38:104–115
Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartiques JF, Alpérovitch A (2007) Dietary patterns and risk of dementia: the Three-City cohort study. Neurology 69:1921–1930
Bartzokis G, Lu PH, Tishler TA, Peters DG, Kosenko A, Barrall KA, Finn JP, Villablanca P, Laub G, Altshuler LL, Geschwind DH, Mintz J, Neely E, Connor JR (2010) Prevalent iron metabolism gene variants associated with increased brain ferritin iron in healthy older men. J Alzheimer’s Dis 20:333–341
Bartzokis G, Lu PH, Tingus K, Peters DG, Amar CP, Tishler TA, Finn JP, Villablanca P, Altshuler LL, Mintz J, Neely E, Connor JR (2011) Gender and iron genes may modify associations between brain iron and memory in healthy aging. Neuropsychopharmacology 36:1375–1384
Benedict C, Jacobsson JA, Rönnemaa E, Sällman-Almén M, Brooks S, Schultes B, Fredriksson R, Lannfelt L, Kilander L, Schiöth HB (2011) The fat mass and obesity gene is linked to reduced verbal fluency in overweight and obese elderly men. Neurobiol Aging 32:1159.e1-5
Bertram L, Heekeren H (2010) Obesity and the brain: a possible genetic link. Alz Res Ther 2:27
Berulava T, Horsthemke B (2010) The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet 18:1054–1056
Bjorkhem I (2006) Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med 260:493–508
Bowman GL, Dodge H, Frei B et al (2009) Ascorbic acid and rates of cognitive decline in Alzheimer’s disease. J Alzheimer’s Dis 16:93–98
Bowman GL, Silbert LC, Howieson D, Dodge HH, Traber MG, Frei B, Kaye JA, Shannon J, Quinn JF (2012) Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology 78:241–249
Brown AA, Hu FB (2001) Dietary modulation of endothelial function: implications for cardiovascular disease. Am J Clin Nutr 73:673–686
Cacabelos R (2003) The application of functional genomics to Alzheimer’s disease. Pharmacogenomics 4:597–621
Cacabelos R (2008) Pharmacogenomics and therapeutic prospects in dementia. Eur Arch Psychiatry Clin Neurosci 258(Suppl 1):28–47
Coppedè F, Tannorella P, Pezzini I, Migheli F, Ricci G, Lenco EC, Piaceri I, Polini A, Nacmias B, Monzani F, Sorbi S, Siciliano G, Migliore L (2011) Folate, homocysteine, vitamin B12, and polymorphisms of genes participating in one-carbon metabolism in late-onset Alzheimer’s disease patients and healthy controls. antioxid redox signal. Dec 15 [Epub ahead of print]
Cronin S, Furie KL, Kelly PJ (2005) Dose-related association of MTHFR 677T allele with risk of ischemic stroke: Evidence from a cumulative meta-analysis. Stroke 36:1581–1587
Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O, Bullido MJ, Engelborghs S, De Deyn P, Berr C, Pasquier F, Dubois B, Tognoni G, Fiévet N, Brouwers N, Bettens K, Arosio B, Coto E, Del Zompo M, Mateo I, Epelbaum J, Frank-Garcia A, Helisalmi S, Porcellini E, Pilotto A, Forti P, Ferri R, Scarpini E, Siciliano G, Solfrizzi V, Sorbi S, Spalletta G, Valdivieso F, Vepsäläinen S, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Hanon O, Piccardi P, Annoni G, Seripa D, Galimberti D, Licastro F, Soininen H, Dartigues JF, Kamboh MI, Van Broeckhoven C, Lambert JC, Amouyel P, Campion D (2011) APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry May 10 [Epub ahead of print]
Gerdes LU, Gerdes C, Kervinen K, Savolainen M, Klausen IC, Hansen PS, Kesäniemi YA, Faergeman O (2000) The apolipoprotein E4 allele determines prognosis and the effect on prognosis of simvastatin in survivors of myocardial infarction. Circulation 101:1366–1371
Ghareeb DA, Hafez HS, Hussien HM, Kabapy NF (2011) Non-alcoholic fatty liver induces insulin resistance and metabolic disorders with development of brain damage and dysfunction. Metab Brain Dis 26:253–267
Giambattistelli F, Bucossi S, Salustri C, Panetta V, Mariani S, Siotto M, Ventriglia M, Vernieri F, Dell’acqua ML, Cassetta E, Rossini PM, Squitti R (2011) Effects of hemochromatosis and transferrin gene mutations on iron dyshomeostasis, liver dysfunction and on the risk of Alzheimer’s disease. Neurobiol Aging Apr 20 [Epub ahead of print]
Gorgone G, Ursini F, Altamura C, Bressi F, Tombini M, Curcio G, Chiovenda P, Squitti R, Silvestrini M, Ientile R, Pisani F, Rossini PM, Vernieri F (2009) Hyperhomocysteinemia, intima-media thickness and C677T MTHFR gene polymorphism: a correlation study in patients with cognitive impairment. Atherosclerosis 206:309–313
Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N (2010) Food combination and Alzheimer disease risk: a protective diet. Arch Neurol 67:699–706
Halsted CH, Villanueva JA, Devlin AM et al (2002) Metabolic interactions of alcohol and folate. J Nutr 132:2367S–2372S
Harwood DG, Kalechstein A, Barker WW, Strauman S, St George-Hyslop P, Iglesias C, Loewenstein D, Duara R (2010) The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. Int J Geriatr Psychiatry 25:511–518
Herrmann W, Obeid R, Schorr H, Zarzour W, Geisel J (2003) Homocysteine, methylenetetrahydrofolate reductase C677T polymorphism and the B-vitamins: a facet of nature-nurture interplay. Clin Chem Lab Med 41:547–553
Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, Dinov ID, Toga AW, Saykin AJ, Shen L, Foroud T, Pankratz N, Huentelman MJ, Craig DW, Gerber JD, Allen AN, Corneveaux JJ, Stephan DA, DeCarli CS, DeChairo BM, Potkin SG, Jack CR Jr, Weiner MW, Raji CA, Lopez OL, Becker JT, Carmichael OT, Thompson PM, Initiative Alzheimer’s Disease Neuroimaging (2010) A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci U S A A107:8404–8409
Holmes MV, Newcombe P, Hubacek JA, Sofat R, Ricketts SL, Cooper J, Breteler MM, Bautista LE, Sharma P, Whittaker JC, Smeeth L, Fowkes FG, Algra A, Shmeleva V, Szolnoki Z, Roest M, Linnebank M, Zacho J, Nalls MA, Singleton AB, Ferrucci L, Hardy J, Worrall BB, Rich SS, Matarin M, Norman PE, Flicker L, Almeida OP, van Bockxmeer FM, Shimokata H, Khaw KT, Wareham NJ, Bobak M, Sterne JA, Smith GD, Talmud PJ, van Duijn C, Humphries SE, Price JF, Ebrahim S, Lawlor DA, Hankey GJ, Meschia JF, Sandhu MS, Hingorani AD, Casas JP (2011) Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet 378:584–594
Hua Y, Zhao H, Kong Y, Ye M (2011) Association between the MTHFR gene and Alzheimer’s disease: a meta-analysis. Int J Neurosci 121:462–471
Hudson JM, Pollux PM, Mistry B, Hobson S (2012) Beliefs about Alzheimer’s disease in Britain. Aging Ment Health. doi:10.1080/13607863.2012.660620
Humphries SE, Talmud PJ, Hawe E, Bolla M, Day IN, Miller GJ (2001) Apolipoprotein E4 and CHD in middle-aged men who smoke: a prospective study. Lancet 358:115–119
Karuppagounder SS, Xu H, Shi Q, Chen LH, Pedrini S, Pechman D, Baker H, Beal MF, Gandy SE, Gibson GE (2009) Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer’s mouse model. Neurobiol Aging 30:1587–1600
Keijzer M, den Heijer BM, Blom HJ, Bos GM, Willems HP, Gerrits WB, Rosendaal FR (2002) Interaction between hyperhomocysteinemia, mutated methylenetetrahydrofolatereductase (MTHFR) and inherited thrombophilic factors in recurrent venous thrombosis. Thromb Haemost 88:723–728
Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C (2011) The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: a prospective cohort study. J Alzheimers Dis 23:461–469
Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, MTHFR Studies Collaboration Group (2002) MTHFR 677C→T polymorphism and risk of coronary heart disease: a meta-analysis. J Am Med Assoc 288:2023–2031
Kotze MJ, Thiart R (2003) Genetics of dyslipidaemia. CME J 21:399–402
Kotze MJ, Davis HJ, Bissbort S, Langenhoven E, Brusnicky J, Oosthuizen CJ (1993a) Intrafamilial variability in the clinical expression of familial hypercholesterolemia: importance of risk factor determination for genetic counselling. Clin Genet 43:295–299
Kotze MJ, de Villiers WJS, Steyn K, Kriek JA, Marais AD, Langenhoven E, Herbert JS, Graadt van Roggen JF, van der Westhuyzen DR, Coetzee GA (1993b) Phenotypic variation among familial hypercholesterolemics heterozygous for either one of two Afrikaner founder LDL receptor mutations. Arterioscler Thromb 13:1460–1468
Kotze MJ, Kriegshäuser G, Thiart R, de Villiers JNP, Scholtz CL, Kury F, Moritz A, Oberkanins C (2003) Simultaneous detection of multiple familial hypercholesterolaemia mutations facilitates an improved diagnostic service in South African patients at high risk of cardiovascular disease. Mol Diagn 7:169–174
Kotze MJ, Thiart R, Hugo FJ, Potocnik FCW (2006) Cardiovascular genetic assessment and treatment in middle age to reduce the risk of heart disease and dementia in old age. SA Fam Pract 48:53–54
Kotze MJ, Van Velden D, Van Rensburg SJ, Erasmus R (2009) Pathogenic mechanisms underlying iron deficiency and iron overload: New insights for clinical application. J Int Fed Clin Chem Lab Med 02–01:1–15
Lehmann DJ, Schuur M, Warden DR, Hammond N, Belbin O, Kölsch H, Lehmann MG, Wilcock GK, Brown K, Kehoe PG, Morris CM, Barker R, Coto E, Alvarez V, Deloukas P, Mateo I, Gwilliam R, Combarros O, Arias-Vásquez A, Aulchenko YS, Ikram MA, Breteler MM, van Duijn CM, Oulhaj A, Heun R, Cortina-Borja M, Morgan K, Robson K, Smith AD (2012) Transferrin and HFE genes interact in Alzheimer’s disease risk: the Epistasis Project. Neurobiol Aging 33(202):e1–e13
Lin M, Zhao L, Fan J, Lian XG, Ye JX, Wu L, Lin H (2011) Association between HFE polymorphisms and susceptibility to Alzheimer’s disease: a meta-analysis of 22 studies including 4,365 cases and 8,652 controls. Mol Biol Rep Jun 24. [Epub ahead of print]
Liu H, Yang M, Li GM, Qiu Y, Zheng J, Du X, Wang JL, Liu RW (2010a) The MTHFR C677T polymorphism contributes to an increased risk for vascular dementia: a meta-analysis. J Neurol Sci 294:74–80
Liu G, Zhu H, Lagou V, Gutin B, Stallmann-Jorgensen IS, Treiber FA, Dong Y, Snieder H (2010b) FTO variant rs9939609 is associated with body mass index and waist circumference, but not with energy intake or physical activity in European- and African-American youth. BMC Med Genet 11:57
Liu Y, Lee SY, Neely E, Nandar W, Moyo M, Simmons Z, Connor JR (2011) Mutant HFE H63D protein is associated with prolonged endoplasmic reticulum stress and increased neuronal vulnerability. J Biol Chem 286:13161–13170
Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB (2005) Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr 135:562–566
Marais AD, Firth JC, Blom DJ (2004) Familial hypercholesterolemia in South Africa. Sem Vasc Med 4:93–95
Matsuzaki T, Sasaki K, Hata J, Hirakawa Y, Fujimi K, Ninomiya T, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T (2011) Association of Alzheimer disease pathology with abnormal lipid metabolism: the Hisayama Study. Neurology 77:1068–1075
Moleres A, Ochoa MC, Rendo-Urteaga T, Martínez-González MA, Azcona San Julián MC, Alfredo Martínez J, Marti A (2011) Dietary fatty acid distribution modifies obesity risk linked to the rs9939609 polymorphism of the fat mass and obesity-associated gene in a Spanish case-control study of children. Br J Nutr 29:1–6
Moriyama Y, Okamura T, Kajinami K, Iso H, Inazu A, Kawashiri M, Mizuno M, Takeda Y, Sakamoto Y, Kimura H, Suzuki H, Mabuchi H (2002) Effects of serum B vitamins on elevated plasma homocysteine levels associated with the mutation of methylenetetrahydrofolate reductase gene in Japanese. Atherosclerosis 164:321–328
Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS (2004) Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology 62:1573–1579
Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC (2006) Trans fatty acids and cardiovascular disease. N Engl J Med 354:1601–1613
Nandar W, Connor JR (2011) HFE gene variants affect iron in the brain. J Nutr 141:729S–739S
Petot GJ, Traore F, Debanne SM, Lerner AJ, Smyth KA, Friedland RP (2003) Interactions of apolipoprotein E genotype and dietary fat intake of healthy older persons during mid-adult life. Metabolism 52:279–281
Poon IO (2008) Effects of antihypertensive drug treatment on the risk of dementia and cognitive impairment. Pharmacotherapy 28:366–375
Potocnik FCV, Bouwens C, van Rensburg SJ (2005) Neurological ageing factors: neurobiology of ageing and prevention of dementia. CME 23:307–309
Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA (1998) Vitamin E suppresses isoprostano generation in vivo and reduces atherosclerosis in Apo E-deficient mice. Nat Med 4:1189–1192
Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F (2005) Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr 82:636–643
Rocchi A, Orsucci D, Tognoni G, Ceravolo R, Siciliano G (2009) The role of vascular factors in late-onset sporadic Alzheimer’s disease. Genetic and molecular aspects. Curr Alzheimer Res 6:224–237
Roest M, van der Schouw YT, de Valk B, Marx JJ, Tempelman MJ, de Groot PG, Sixma JJ, Banga JD (1999) Heterozygosity for a hereditary hemochromatosis gene is associated with cardiovascular death in women. Circulation 100:1268–1273
Romero LJ, Garry PJ, Schuyler M, Bennahum DA, Qualls C, Ballinger L, Kelly V, Schmitt C, Skipper B, Ortiz IE, Rhyne RL (2005) Emotional responses to APO E genotype disclosure for Alzheimer disease. J Genet Couns 14:141–150
Rondeau V, Iron A, Letenneur L, Commenges D, Duchêne F, Arveiler B, Dartigues JF (2006) Analysis of the effect of aluminum in drinking water and transferrin C2 allele on Alzheimer’s disease. Eur J Neurol 13:1022–1025
Sampietro M, Caputo L, Casatta A, Meregalli M, Pellagatti A, Tagliabue J, Annoni G, Vergani C (2001) The hemochromatosis gene affects the age of onset of sporadic Alzheimer’s disease. Neurobiol Aging 22:563–568
Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA (2006) Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 59:912–921
Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA (2006) Plasma phosphatidylcholine, docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 63:1545–1550
Seneff S, Wainwright G, Mascilelli L (2011) Nutrition and Alzheimer’s disease: the detrimental role of a high carbohydrate diet. Eur J Intern Med 22:134–140
Seshadri S, Drachman DA, Lippa CF (1995) Apolipoprotein E E4 allele and the lifetime risk of Alzheimer’s disease. What physicians know, and what they should know. Arch Neurol 52:1074–1079
Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA (2002) Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346:476–483
Shmeleva V, Kapustin MSI, Papayan LP, Sobczyńska-Malefora A, Harrington DJ, Savidge GF (2003) Prevalence of hyperhomocysteinemia and the MTHFR C677T polymorphism in patients with arterial and venous thrombosis from North Western Russia. Thromb Res 111:351–356
Siuda J, Gorzkowska A, Patalong-Ogiewa M, Krzystanek E, Czech E, Wiechuła B, Garczorz W, Danch A, Jasińska-Myga B, Opala G (2009) From mild cognitive impairment to Alzheimer’s disease—influence of homocysteine, vitamin B12 and folate on cognition over time: results from one-year follow-up. Neurol Neurochir Pol 43:321–329
Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA (2009) Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord 28:75–80
Styczyńska M, Strosznajder JB, Religa D, Chodakowska-Zebrowska M, Pfeffer A, Gabryelewicz T, Czapski GA, Kobryś M, Karciauskas G, Barcikowska M (2008) Association between genetic and environmental factors and the risk of Alzheimer’s disease. Folia Neuropathol 46:249–254
Szolnoki Z, Somogyvari F, Kondacs A, Szobo M, Fodor L, Bene J, Melegh B (2003) Evaluation of the modifying effects of unfavourable genetypes on classical clinical risk factors for ischaemic stroke. J Neurol Neurosurg Psychiatry 74:1615–1620
Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH (2008) B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci USA 105:12474–12479
Van Rensburg SJ, Carstens ME, Potocnik FC, Aucamp AK, Taljaard JJ (1993) Increased frequency of the transferrin C2 subtype in Alzheimer’s disease. Neuroreport 4:1269–1271
Van Rensburg SJ, Potocnik FCV, de Villiers JNP, Kotze MJ, Taljaard JJF (2000) Earlier age of onset in Alzheimer’s disease patients with both the transferrin C2 and apolipoprotein E4 alleles. Ann N Y Acad Sci 903:200–203
Van Rensburg SJ, Potocnik FCV, Kotze MJ, Stein DJ (2010) Antemortem Markers. In: Abou-Saleh MT, Katona CLE, Kumar A (eds) Principles and practice of geriatric psychiatry, 3rd edn. Wiley, Sussex, pp 299–303
Van Velden DP, van der Merwe S, Fourie E, Blackhurst DM, Kidd M, Kotze MJ, Mansvelt EPG (2007) The influence of a Mediterranean-like diet with and without red wine on patients with the metabolic syndrome. S Afr J Enol Vitic 28:44–49
Vergotine J, Thiart R, Scholtz CL, Kotze MJ (2001) Clinical versus molecular diagnosis of heterozygous familial hypercholesterolaemia in the South African population. S Afr Med J 91:1053–1059
Volzke H, Wolff B, Grimm R, Robinson DM, Sxhuster G, Herrmann FH, Motz W, Rettig R (2005) Interaction between factor V Leiden and serum LDL cholesterol increases the risk of atherosclerosis. Atherosclerosis 180:341–347
Wald DS, Law M, Morris JK (2002) Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Br Med J 325:1202
Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr, Yaffe K (2005a) Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Br Med J 330:1360
Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005b) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64:277–281
World Health Organisation: Joint effects of risk factors. http://www.who.int/healthinfo/global_burden_disease/global_health_risks/en/index.html. Accessed 14 March 2012
Zambenedetti P, De Bellis G, Biunno I, Musicco M, Zatta P (2003) Transferrin C2 variant does confer a risk for Alzheimer’s disease in caucasians. J Alzheimers Dis 5:423–427
Zhang MY, Miao L, Li YS, Hu GY (2010) Meta-analysis of the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to Alzheimer’s disease. Neurosci Res 68:142–150
Acknowledgments
The authors gratefully acknowledge the financial support provided by Winetech, the Technology for Human Resources and Industry Program (THRIP), the South African Medical Research Council, the Harry Crossley Foundation and the National Research Foundation (NRF disclaimer: Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regards thereto). Yandiswa Yako, Mahjoubah Jalali, Leslie Fisher and Kobus Pretorius are thanked for their contributions to the implementation of an improved diagnostic service for cardiovascular disease in the South African population. Dr Maria Christodoulou, Programme Manager for Integrative Medicine, University of Stellenbosch, is acknowledged for supporting clinical application in a postgraduate curriculum. Professors Johann Schneider and Rajiv Erasmus are thanked for their support.
Conflict of interest
Prof MJ Kotze is a director and shareholder of Gknowmix (Pty) Ltd. that developed a database tool for conversion of research and innovation under the auspices of the Innovation Centre of the South African Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kotze, M.J., van Rensburg, S.J. Pathology supported genetic testing and treatment of cardiovascular disease in middle age for prevention of Alzheimer’s disease. Metab Brain Dis 27, 255–266 (2012). https://doi.org/10.1007/s11011-012-9296-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-012-9296-8