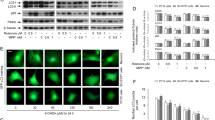
Abstract
At the neuropathological level, Parkinson’s disease (PD) is characterized by the accumulation of misfolded proteins, which can trigger the unfolded protein response (UPR). UCH-L1 is a component of ubiquitin proteasome system (UPS). It is reported that the loss of its function will impair ubiquitin proteasome system and cause toxicity to cells. But its mechanism has not been illustrated. In this study, we detected the protein expression of Bip/Grp78 and the spliced form of XBP-1 to examine the activation of unfolded protein response after SK-N-SH cells being treated with LDN-57444, a UCH-L1 inhibitor which could inhibit UCH-L1 hydrolase activity. Our data showed that UCH-L1 inhibitor was able to cause cell death through the apoptosis pathway by decreasing the activity of ubiquitin proteasome system and increasing the levels of highly ubiquitinated proteins, both of which can activate unfolded protein response. There is a lot of evidence that unfolded protein response is activated as a protective response at the early stage of the stress; this protective response can switch to a pro-apoptotic response when the stress persists. In this study, we demonstrated this switch by detecting the upregulation of CHOP/Gadd153. Taken together, our data indicated that the apoptosis induced by UCH-L1 inhibitor may be triggered by the activation of endoplasmic reticulum stress (ERS). Moreover, we provide a new cell model for studying the roles of UCH-L1 in Parkinson’s disease.




Similar content being viewed by others
References
Wilkinson KD, Lee KM, Deshpande S et al (1989) The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science 246:670–673. doi:10.1126/science.2530630
Leroy E, Boyer R, Auburger G et al (1998) The ubiquitin pathway in Parkinson’s disease. Nature 395:451–452. doi:10.1038/26652
Nishikawa K, Li H, Kawamura R et al (2003) Alterations of structure and hydrolase activity of parkinsonism-associated human ubiquitin carboxyl-terminal hydrolase L1 variants. Biochem Biophys Res Commun 304:176–183. doi:10.1016/S0006-291X(03)00555-2
Hoozemans JJ, van Haastert ES, Eikelenboom P et al (2007) Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun 354:707–711. doi:10.1016/j.bbrc.2007.01.043
Holtz WA, O’Malley KL (2003) Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem 278:19367–19377. doi:10.1074/jbc.M211821200
Ryu EJ, Harding HP, Angelastro JM et al (2002) Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci 22:10690–10698
Imai Y, Soda M, Inoue H et al (2001) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105:891–902. doi:10.1016/S0092-8674(01)00407-X
Cooper AA, Gitler AD, Cashikar A et al (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313:324–328. doi:10.1126/science.1129462
McNaught KS, Mytilineou C, Jnobaptiste R et al (2002) Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J Neurochem 81:301–306. doi:10.1046/j.1471-4159.2002.00821.x
Liu Y, Lashuel HA, Choi S et al (2003) Discovery of inhibitors that elucidate the role of UCH-L1 activity in the H1299 lung cancer cell line. Chem Biol 10:837–846. doi:10.1016/j.chembiol.2003.08.010
Gong B, Cao ZX, Zheng P (2006) Ubiquitin hydrolase Uch-l1 rescues β-Amyloid-induced decreases in synaptic function and contextual memory. Cell 126:775–788. doi:10.1016/j.cell.2006.06.046
Fujimuro M, Sawada H, Yokosawa H (1994) Production and characterization of monoclonal antibodies specific to multi-ubiquitin chains of polyubiquitinated proteins. FEBS Lett 349:173–180. doi:10.1016/0014-5793(94)00647-4
Yoshida H, Haze K, Yanagi H et al (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273:33741–33749. doi:10.1074/jbc.273.50.33741
Szegezdi E, Fitzgerald U, Samali A (2003) Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci 1010:186–194. doi:10.1196/annals.1299.032
McCullough KD, Martindale JL, Klotz LO et al (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259. doi:10.1128/MCB.21.4.1249-1259.2001
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459. doi:10.1128/MCB.23.21.7448-7459.2003
Plongthongkum N, Kullawong N, Panyim S et al (2007) Ire1 regulated XBP1 mRNA splicing is essential for the unfolded protein response (unfolded protein response) in Drosophila melanogaster. Biochem Biophys Res Commun 354:789–794. doi:10.1016/j.bbrc.2007.01.056
Yoshida H, Matsui T, Yamamoto A et al (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891. doi:10.1016/S0092-8674(01)00611-0
Lee K, Tirasophon W, Shen X et al (2002) IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16:452–466. doi:10.1101/gad.964702
Ciechanover A, Brundin P (2003) The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40:427–446. doi:10.1016/S0896-6273(03)00606-8
Akiko Y, Yasuhiro Y, Kiyokazu O et al (2006) Involvement of endoplasmic reticulum stress on cell death induced by 6-OHDA. Neurochem Res 31:657–664. doi:10.1007/s11064-006-9062-6
Rutkowski DT, Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14:20–28. doi:10.1016/j.tcb.2003.11.001
Pelham HR (1989) Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol 5:1–23. doi:10.1146/annurev.cb.05.110189.000245
Wang XZ, Kuroda M, Sok J et al (1998) Identification of novel stress-induced genes downstream of chop. EMBO J 17:3619–3630. doi:10.1093/emboj/17.13.3619
Bennett MC, Bishop JF, Leng Y et al (1999) Degradation of alpha-synuclein by proteasome. J Biol Chem 274:33855–33858. doi:10.1074/jbc.274.48.33855
Rideout HJ, Larsen KE, Sulzer D et al (2001) Proteasomal inhibition leads to formation of ubiquitin/alpha-synuclein-immunoreactive inclusions in PC12 cells. J Neurochem 78:899–908. doi:10.1046/j.1471-4159.2001.00474.x
Lee HJ, Suk JE, Bae EJ et al (2008) Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol; Epub ahead of print
Acknowledgments
This work was supported by grants from the National Program of Basic Research (2006CB500706) of China, National Natural Science Fund (30471918, 30570637), Shanghai Key Project of Basic Science Research (04DZ14005) and Program for Outstanding Medical Academic Leader (LJ 06003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, YY., Zhou, HY., Wang, ZQ. et al. Endoplasmic reticulum stress contributes to the cell death induced by UCH-L1 inhibitor. Mol Cell Biochem 318, 109–115 (2008). https://doi.org/10.1007/s11010-008-9862-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9862-x