Abstract
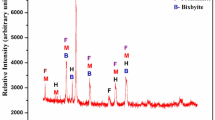
Coal is an important fuel used in boiler furnaces. There are problems like unburnt coal and solid wastes like ash contain arsenic, selenium, chromium and cadmium while using it. In order to avoid all such difficulties, aluminium metal powder in various grain sizes mixed with pulverized coal and burned. aluminium metal powder is one of the pyrotechnics having higher calorific value and low ignition temperature. The thermal behavior of aluminium powder along with coal is recorded in DTA. The collected ashes were tested in Scanning Electron Microscope and X-Ray Diffraction meter. The SEM results show that coal ash is having granular and regular structure. All the particles have a size range from 5 to 8 μm. On the other hand, the aluminium coal mixture ash shows a fibrous matrix and the particles are irregular. In XRD graph, the peaks in the graph show orientation of atoms in particular plane and angle. The coal ash has a lot of peaks, but the maximum count value reaches only to 340.97. However the value of counts reaches a maximum of 1,539.06 for aluminium ash. This denotes high atom orientation in a single lattice plane.










Similar content being viewed by others
References
Special article, Metal powder hazards, LPA 4 (2004), 22.
Azhagurajan A. Enhancement of explosive property of nano scale pyro aluminium powder. In: Proceedings international conference on nano materials, MSEC. 2005. ISBN p. 383–87.
Ghosh KN. The principles of fireworks. In: Khastsuria H, editor.; 1987. p. 52.
Lakshmanan K. Chemistry of fire works. In: Proceedings national seminar on Fireworks Safety 99, MSEC: Vikas; 1999. p. 23.
Roth G. Performance of explosives, German patent; No. 1,733,271,900; 2000.
Maranda A. Research on the process of detonation of explosive of mixtures of the oxidizer fuel type containing aluminium powder. Propell Explos Pyrotech. 1990;15:161–65.
Thomas K, Andreas H, Alois K. Influence of aluminium/ammonium perchlorate on the performance of underwater explosives. Propell Explos Pyrotech. 1999;24:140–43.
Gharia JS, Athawale BK, Visal SR, Phadthare VV. Effect of aluminium particle size and percentage on blast compositions, Symposium on Warhead Technology, TBRL: Chandigarh; 1983. p. 144.
Stromose E, Eriksen SW. Performance of high explosives in underwater application, part 2: aluminised explosives. Propell Explos Pyrotech. 1990;15:52–53.
Vinay P. Influence of alumnium on performance of HTPB-based aluminised PBXs. Def Sci J. 2004;4:475.
http://www.argonide.com/propellants.html. Nano aluminum powder for medium-caliber projectiles, accelerator or as a booster.
Mukunda HS. Understanding Combustion, Macmillan India Ltd; 1989. p 22.
Acknowledgements
A. Azhagurajan thanks Head, Department of Mechanical Engineering and Principal, Mepco Schlenk Engineering College for providing financial support to test the chemicals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azhagurajan, A., Nagaraj, P. An experimental analysis of coal aluminium mixture in coal fired furnace. J Therm Anal Calorim 98, 253–259 (2009). https://doi.org/10.1007/s10973-009-0009-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0009-4