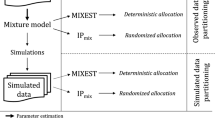
Mixture models are applied in population pharmacometrics to characterize underlying population distributions that are not adequately approximated by a single normal or lognormal distribution. In addition to obtaining individualized maximum a posteriori Bayesian post hoc parameter estimates, the subpopulation to which an individual was classified can be determined. However, the accuracy of the classification of subjects to subpopulations is not well studied. We investigated the impact of several factors on the accuracy of classification in mixture models applied to pharmacokinetics using a simulation strategy. The availability of actual subject data allowed us to evaluate mixture model classification in a potentially common application, namely, the classification of clearance into poor metabolizer (PM) or extensive metabolizer (EM) subgroups with the known phenotype status in subjects receiving metoprolol. The factors explored in the simulation study were the magnitude of difference between the clearances in two subpopulations, the between subject variability in clearance, the mixing-fraction, and the population sample size. Populations were simulated at various levels of the above factors and analyzed with a mixture model using NONMEM. The population pharmacokinetics of metoprolol were modeled with the EM/PM phenotype as a known covariate, and without the phenotype covariate using a mixture model. Within the range of scenarios studied, the proportion of subjects classified into the correct subpopulation was high. The simulation–estimation study suggests that a greater separation between two subpopulations, a smaller variability in the parameter distribution, a larger sample size, and a smaller size subpopulation tend to be associated with a greater accuracy of subpopulation classification when a mixture model is applied to pharmacokinetic data. In a population pharmacokinetic analysis of metoprolol, a drug that undergoes polymorphic metabolism, it was possible to correctly identify phenotype status using a mixture model.
Similar content being viewed by others
References
Hussein R., Charles B.G., Morris R.G., Rasiah R.L. (2001) Population pharmacokinetics of perhexiline from very sparse, routine monitoring data. Ther. Drug Monit. 23:636–643
Frame B., Miller R., Lalonde R.L. (2003) Evaluation of mixture modeling with count data using NONMEM. J. Pharmacokinet. Pharmacodyn. 30:167–183
Facca B., Frame B., Triesenberg S. (1998) Population pharmacokinetics of ceftizoxime administered by continuous infusion in clinically ill adult patients. Antimicrob. Agents Chemother. 42:1783–1787
Borg K.O. et al. (1975) Metabolism of metoprolol-(3-h) in man, the dog and the rat. Acta Pharmacol. Toxicol. (Copenh). 36(Suppl):125–135
Otton S.V., Crewe H.K., Lennard M.S., Tucker G.T., Woods H.F. (1988) Use of quinidine inhibition to define the role of the sparteine/debrisoquine cytochrome P450 in metoprolol oxidation by human liver microsomes. J. Pharmacol. Exp. Ther. 247:242–247
Johnson J.A., Burlew B.S. (1996). Metoprolol metabolism via cytochrome P4502D6 in ethnic populations. Drug Metab. Dispos. 24:350–355
http://www.imm.ki.se/CYPalleles/. Human cytochrome P450 (CYP) allele nomenclature committee. Criteria for inclusion, and nomenclature files [online].
Streetman D.S., Bertino J.S. Jr., Nafziger A.N. (2000) Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics 10:187–216
Alvan G., Bechtel P., Iselius L., Gundert-Remy U. (1990) Hydroxylation polymorphisms of debrisoquine and mephenytoin in European populations. Eur. J. Clin. Pharmacol. 39:533–537
Bertilsson L., Dahl M.L. (1996) Polymorphic drug oxidation: relevance to the treatment of psychiatric disorders. CNS Drugs 5:200–223
Bertilsson L. et al. (1992) Pronounced differences between native Chinese and Swedish populations in the polymorphic hydroxylations of debrisoquin and S-mephenytoin. Clin. Pharmacol. Ther. 51:388–397
Evans D.A., Mahgoub A., Sloan T.P., Idle J.R., Smith R.L. (1980) A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J. Med. Genet. 17:102–105
Johansson I. et al. (1993) Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc. Natl. Acad. Sci. USA 90:11825–11829
Dahl M.L., Johansson I., Palmertz M.P., Ingelman-Sundberg M., Sjoqvist F. (1992) Analysis of the CYP2D6 gene in relation to debrisoquin and desipramine hydroxylation in a Swedish population. Clin. Pharmacol. Ther. 51:12–17
Dahl M.L., Johansson I., Bertilsson L., Ingelman-Sundberg M., Sjoqvist F. (1995) Ultrarapid hydroxylation of debrisoquine in a Swedish population. Analysis of the molecular genetic basis. J. Pharmacol. Exp. Ther. 274:516–520
Wikstrand J. et al. (1988) Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study. JAMA 259:1976–1982
Straka R.J., Johnson K.A., Gross C.R., Brundage R.C., McBride J.W. (1991) Pharmacodynamic modeling of metoprolol enantiomers in poor and extensive metabolizers of debrisoquin. Clin. Pharmacol. Ther. 49:157
Straka R.J., Marshall P.S., Johnson K.A., Carr J.C., McBride J.W. (1992) The effects of smoking on post exercise heart rate and metoprolol enantiomeric pharmacodynamics in extensive metabolisers of dextromethorphan. Pharmacotherapy 12(3):1992
Straka R.J., Johnson K.A., Marshall P.S., Remmel R.P. (1990) Analysis of metoprolol enantiomers in human serum by liquid chromatography on a cellulose-based chiral stationary phase. J. Chromatogr. 530:83–93
Straka R.J., Hansen S.R., Walker P.F. (1995) Comparison of the prevalence of the poor metabolizer phenotype for CYP2D6 between 203 Hmong subjects and 280 white subjects residing in Minnesota. Clin. Pharmacol. Ther. 58:29–34
Kirchheiner J. et al. (2004) Impact of the ultrarapid metabolizer genotype of cytochrome P450 2D6 on metoprolol pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 76:302–312
Zineh I. et al. (2004) Pharmacokinetics and CYP2D6 genotypes do not predict metoprolol adverse events or efficacy in hypertension. Clin. Pharmacol. Ther. 76:536–544
Schaaf L.J., Dobbs B.R., Edwards I.R., Perrier D.G. (1987) Nonlinear pharmacokinetic characteristics of 5-fluorouracil (5-FU) in colorectal cancer patients. Eur. J. Clin. Pharmacol. 32:411–418
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaila, N., Straka, R.J. & Brundage, R.C. Mixture Models and Subpopulation Classification: A Pharmacokinetic Simulation Study and Application to Metoprolol CYP2D6 Phenotype. J Pharmacokinet Pharmacodyn 34, 141–156 (2007). https://doi.org/10.1007/s10928-006-9038-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-006-9038-9