Abstract
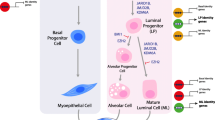
Most of the development and functional differentiation in the mammary gland occur after birth. Epigenetics is defined as the stable alterations in gene expression potential that arise during development and proliferation. Epigenetic changes are mediated at the biochemical level by the chromatin conformation initiated by DNA methylation, histone variants, post-translational modifications of histones, non-histone chromatin proteins, and non-coding RNAs. Epigenetics plays a key role in development. However, very little is known about its role in the developing mammary gland or how it might integrate the many signalling pathways involved in mammary gland development and function that have been discovered during the past few decades. An inverse relationship between marks of closed (DNA methylation) or open chromatin (DnaseI hypersensitivity, certain histone modifications) and milk protein gene expression has been documented. Recent studies have shown that during development and functional differentiation, both global and local chromatin changes occur. Locally, chromatin at distal regulatory elements and promoters of milk protein genes gains a more open conformation. Furthermore, changes occur both in looping between regulatory elements and attachment to nuclear matrix. These changes are induced by developmental signals and environmental conditions. Additionally, distinct epigenetic patterns have been identified in mammary gland stem and progenitor cell sub-populations. Together, these findings suggest that epigenetics plays a role in mammary development and function. With the new tools for epigenomics developed in recent years, we now can begin to establish a framework for the role of epigenetics in mammary gland development and disease.





Similar content being viewed by others
Abbreviations
- BCE:
-
beta casein enhancer
- C/EBPβ:
-
CCAAT-enhancer-binding proteins
- ChIP:
-
chromatin immunoprecipitation
- DNAme:
-
DNA methylation
- DHS:
-
DNaseI hypersensitivity
- DRE:
-
distal regulatory elements
- ECR:
-
evolutionary conserved regions
- EMSA:
-
Electro Mobility Shift Assay
- Gc:
-
glucocorticoid
- GR:
-
glucocorticoid receptor
- MEC:
-
mammary epithelial cell
- Pg:
-
progesterone
- PR:
-
progesterone receptor
- Prl:
-
prolactin
- ncRNA:
-
non-coding RNA
- STAT5:
-
signal transducers and activators of transcription
References
Topper YJ, Freeman CS. Multiple interactions in the developmental biology of the mammary gland. Physiol Rev. 1980;80:1049–56.
Stein T, Morris JS, Davies CR, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–91.
Master SR, Stoddard AJ, Bailey LC, Pan TC, Dugan KD, Chodosh LA. Genomic analysis of early murine mammary gland development using novel probe-level algorithms. Genome Biol. 2005;6:R20.
McBryan J, Howlin J, Kenny PA, Shioda T, Martin F. ERalpha-CITED1 co-regulated genes expressed during pubertal mammary gland development: implications for breast cancer prognosis. Oncogene. 2007;26:6406–19.
Rudolph MC, McManaman JL, Phang T, et al. Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol Genomic. 2007;28:323–36.
Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6:R92–109.
Kendrick H, Regan JL, Magnay FA, et al. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics. 2008;9:591.
Raouf A, Zhao Y, To K, et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell. 2008;3:109–18.
Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–77.
Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135:995–1003.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54.
Okano H, Temple S. Cell types to order: temporal specification of CNS stem cells. Curr Opin Neurobiol. 2009;19:112–9.
Haumaitre C, Lenoir O, Scharfmann R. Directing cell differentiation with small-molecule histone deacetylase inhibitors: the example of promoting pancreatic endocrine cells. Cell Cycle. 2009;8:536–44.
Waterland RA, Kellermayer R, Rached MT, et al. Epigenomic profiling indicates a role for DNA methylation in early postnatal liver development. Hum Mol Genet. 2009;18:3026–38.
Bromfield J, Messamore W, Albertini DF. Epigenetic regulation during mammalian oogenesis. Reprod Fertil Dev. 2008;20:74–80.
Broske AM, Vockentanz L, Kharazi S, et al. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet. 2009;41:1207–15.
Cui K, Zang C, Roh TY, et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93.
Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105.
Reiner SL. Epigenetic control in the immune response. Hum Mol Genet. 2005;14(Spec No 1):R41–6.
Janson PC, Winerdal ME, Winqvist O. At the crossroads of T helper lineage commitment-epigenetics points the way. Biochim Biophys Acta. 2009;1790:906–19.
Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–3.
Dobson H, Smith R, Royal M, Knight C, Sheldon I. The high-producing dairy cow and its reproductive performance. Reprod Domest Anim. 2007;42 Suppl 2:17–23.
Bobe G, Lindberg GL, Reutzel LF, Hanigan MD. Effects of lipid supplementation on the yield and composition of milk from cows with different beta-lactoglobulin phenotypes. J Dairy Sci. 2009;92:197–203.
Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–402.
Bernstein E, Hake SB. The nucleosome: a little variation goes a long way. Biochem Cell Biol. 2006;84:505–17.
Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705.
Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–99.
Dhasarathy A, Wade PA. The MBD protein family-reading an epigenetic mark? Mutat Res. 2008;647:39–43.
Postnikov Y, Bustin M. Regulation of chromatin structure and function by HMGN proteins. Biochim Biophys Acta. 2009.
Mattick JS, Amaral PP, Dinger ME, Mercer TR, Mehler MF. RNA regulation of epigenetic processes. Bioessays. 2009;31:51–9.
Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell. 2002;10:1453–65.
de Laat W, Grosveld F. Spatial organization of gene expression: the active chromatin hub. Chromosome Res. 2003;11:447–59.
Marzluff Jr WF, McCarty KS. Two classes of histone acetylation in developing mouse mammary gland. J Biol Chem. 1970;245:5635–42.
Hohmann P, Cole RD. Hormonal effects on amino acid incorporation into lysine-rich histones in the mouse mammary gland. J Mol Biol. 1971;58:33–540.
Crawford GE, Davis S, Scacheri PC, et al. DNase-chip: a high-resolution method to identify DNase I hypersensitive sites using tiled microarrays. Nat Methods. 2006;3:503–9.
Sabo PJ, Kuehn MS, Thurman R, et al. Genome-scale mapping of DNase I sensitivity in vivo using tiling DNA microarrays. Nat Methods. 2006;3:511–8.
Clark SJ, Harrison J, Paul CL, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22:2990–7.
Wilson IM, Davies JJ, Weber M, et al. Epigenomics: mapping the methylome. Cell Cycle. 2006;5:155–8.
Lister R, Ecker JR. Finding the fifth base: genome-wide sequencing of cytosine methylation. Genome Res. 2009;19:959–66.
Johnson KD, Bresnick EH. Dissecting long-range transcriptional mechanisms by chromatin immunoprecipitation. Methods. 2002;26:27–36.
Kirmizis A, Bartley SM, Kuzmichev A, et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18:1592–605.
Barski A, Zhao K. Genomic location analysis by ChIP-Seq. J Cell Biochem. 2009;107:11–8.
Waalwijk C, Flavell RA. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978;5:4631–4.
Waalwijk C, Flavell RA. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978;5:3231–6.
Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–32.
Groudine M, Kohwi-Shigematsu T, Gelinas R, Stamatoyannopoulos G, Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the beta-globin gene locus. Proc Natl Acad Sci U S A. 1983;80:7551–5.
Forrester WC, Thompson C, Elder JT, Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci. 1986;83:1359–63.
Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp Cell Res. 2004;298:122–32.
Johnson ML, Levy J, Supowit SC, Yu-Lee LY, Rosen JM. Tissue- and cell-specific casein gene expression. II. Relationship to site-specific DNA methylation. J Biol Chem. 1983;258:10805–11.
Thompson MD, Nakhasi HL. Methylation and expression of rat kappa-casein gene in normal and neoplastic rat mammary gland. Cancer Res. 1985;45:1291–5.
Platenburg GJ, Vollebregt EJ, Karatzas CN, Kootwijk EPA, de Boer HA, Strijker R. Mammary gland-specific hypomethylation of hpaii sites flanking the bovine alpha-s1-casein gene. Transgenic Res. 1996;5:421–31.
Vanselow J, Yang W, Herrmann J, et al. DNA-remethylation around a STAT5-binding enhancer in the alphaS1-casein promoter is associated with abrupt shutdown of alphaS1-casein synthesis during acute mastitis. J Mol Endocrinol. 2006;37:463–77.
Singh K, Swanson K, Couldrey C, Seyfert H-M, Stelwagen K. Suppression of bovine αS1-casein gene expression during involution of the mammary gland is associated with increased DNA methylation at a STAT5-binding site in the αS1-casein promoter. J Dairy Sci. 2008;91:378.
Rijnkels M, Freeman-Zadrowski C, Hernandez J. Epigenetic changes during functional differentiation of the mammary gland. J Dairy Sci. 2009;92:325–6.
Dandekar AM, Robinson EA, Appella E, Qasba PK. Complete sequence analysis of cDNA clones encoding rat whey phosphoprotein: homology to a protease inhibitor. Proc Natl Acad Sci U S A. 1982;79:3987–91.
Hennighausen LG, Sippel AE. Characterization and cloning of the mRNAs specific for the lactating mouse mammary gland. Eur J Biochem. 1982;125:131–41.
Montazer-Torbati MB, Hue-Beauvais C, Droineau S, et al. Epigenetic modifications and chromatin loop organization explain the different expression profiles of the Tbrg4, WAP and Ramp3 genes. Exp Cell Res. 2008;314:975–87.
Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–8.
Denamur R. Nucleic acids of the mammary gland during gestation and lactation in the rabbit. C R Hebd Seances Acad Sci. 1963;256:4748–50.
Traurig HH. Cell proliferation in the mammary gland during late pregnancy and lactation. Anat Rec. 1967;157:489–503.
Traurig HH. A radioautographic study of cell proliferation in the mammary gland of the pregnant mouse. Anat Rec. 1967;159:239–47.
Whitelaw CBA, Harris S, McClenaghan M, Simons JP, Clark AJ. Position-independent expression of the ovine beta-lactoglobin gene in transgenic mice. Biochem J. 1992;286:31–9.
Whitelaw C. Hormonal influences on beta-lactoglobulin transgene expression inferred from chromatin structure. Biochem Biophys Res Commun. 1996;224:121–5.
Whitelaw CB. Nucleosome organisation of the beta-lactoglobulin gene. Transcription complex formation. Adv Exp Med Biol. 2000;480:147–53.
Whitelaw CB, Webster J. Temporal profiles of appearance of DNase I hypersensitive sites associated with the ovine beta-lactoglobulin gene differ in sheep and transgenic mice. Mol Gen Genet. 1998;257:649–54.
Barber MC, Vallance AJ, Kennedy HT, Travers MT. Induction of transcripts derived from promoter III of the acetyl-CoA carboxylase-alpha gene in mammary gland is associated with recruitment of SREBP-1 to a region of the proximal promoter defined by a DNase I hypersensitive site. Biochem J. 2003;375:489–501.
Li S, Rosen JM. Glucocorticoid regulation of rat whey acidic protein gene expression involves hormone-induced alterations of chromatin structure in the distal promoter region. Mol Endocrinol. 1994;8:1328–35.
Millot B, Fontaine ML, Thepot D, Devinoy E. A distal region, hypersensitive to DNase I, plays a key role in regulating rabbit whey acidic protein gene expression. Biochem J. 2001;359:557–65.
Millot B, Montoliu L, Fontaine ML, Mata T, Devinoy E. Hormone-induced modifications of the chromatin structure surrounding upstream regulatory regions conserved between the mouse and rabbit whey acidic protein genes. Biochem J. 2003;372:41–52.
Rijnkels M, Elnitski L, Miller W, Rosen JM. Multi-species comparative analysis of a mammalian specific genomic domain encoding secretory proteins. Genomics. 2003;82:417–32.
Hebbes TR, Thorne AW, Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988;7:1395–402.
Roh TY, Ngau WC, Cui K, Landsman D, Zhao K. High-resolution genome-wide mapping of histone modifications. Nat Biotechnol. 2004;22:1013–6.
Heintzman ND, Hon GC, Hawkins RD, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–12.
Heintzman ND, Stuart RK, Hon G, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–8.
Jolivet G, Pantano T, Houdebine LM. Regulation by the extracellular matrix (ECM) of prolactin-induced alphas1-casein gene expression in rabbit primary mammary cells: Role of STAT5, C/EBP, and chromatin structure. J Cell Biochem. 2005;95:313–27.
Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–11.
Simonis M, Klous P, Splinter E, et al. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat Genet. 2006;38:1348–54.
Zhao Z, Tavoosidana G, Sjolinder M, et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet. 2006;38:1341–7.
Dostie J, Richmond TA, Arnaout RA, et al. Chromosome Conformation Capture Carbon Copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006;16:1299–309.
Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The beta-globin nuclear compartment in development and erythroid differentiation. Nat Genet. 2003;35:190–4.
Jiang H, Peterlin BM. Differential chromatin looping regulates CD4 expression in immature thymocytes. Mol Cell Biol. 2008;28:907–12.
Vernimmen D, De Gobbi M, Sloane-Stanley JA, Wood WG, Higgs DR. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007;26:2041–51.
Kabotyanski EB, Rijnkels M, Freeman-Zadrowski C, Buser AC, Edwards DP, Rosen JM. Lactogenic hormonal induction of long-distance interactions between {beta}-casein gene regulatory elements. J Biol Chem. 2009;284:22815–24.
Myers CA, Schmidhauser C, Mellentin-Michelotti J, et al. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol Cell Biol. 1998;18:2184–95.
Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of beta-casein gene expression. Mol Biol Cell. 1992;3:699–709.
Winklehner-Jennewein P, Geymayer S, Lechner J, et al. A distal enhancer region in the human beta-casein gene mediates the response to prolactin and glucocorticoid hormones. Gene. 1998;217:127–39.
Buser AC, Gass-Handel EK, Wyszomierski SL, et al. Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the beta-casein gene in mammary epithelial cells. Mol Endocrinol. 2007;21:106–25.
Vassetzky Y, Lemaitre JM, Mechali M. Specification of chromatin domains and regulation of replication and transcription during development. Crit Rev Eukaryot Gene Exp. 2000;10:31–8.
Eivazova ER, Gavrilov A, Pirozhkova I, et al. Interaction in vivo between the two matrix attachment regions flanking a single chromatin loop. J Mol Biol. 2009;386:929–37.
Razin SV, Iarovaia OV, Sjakste N, et al. Chromatin domains and regulation of transcription. J Mol Biol. 2007;369:597–607.
Ballester M, Kress C, Hue-Beauvais C, et al. The nuclear localization of WAP and CSN genes is modified by lactogenic hormones in HC11 cells. J Cell Biochem. 2008;105:262–70.
Ioudinkova E, Petrov A, Razin SV, Vassetzky YS. Mapping long-range chromatin organization within the chicken alpha-globin gene domain using oligonucleotide DNA arrays. Genomics. 2005;85:143–51.
Brisken C, Rajaram RD. Alveolar and lactogenic differentiation. J Mammary Gland Biol Neoplasia. 2006;11:239–48.
Groner B. Transcription factor regulation in mammary epithelial cells. Domest Anim Endocrinol. 2002;23:25–32.
Doppler W, Geymayer S, Weirich HG. Synergistic and antagonistic interactions of transcription factors in the regulation of milk protein gene expression. Mechanisms of cross-talk between signalling pathways. Adv Exp Med Biol. 2000;480:139–46.
Kabotyanski EB, Huetter M, Xian W, Rijnkels M, Rosen JM. Integration of prolactin and glucocorticoid signaling at the {beta}-casein promoter and enhancer by ordered recruitment of specific transcription factors and chromatin modifiers. Mol Endocrinol. 2006;20:2355–68.
Xu R, Nelson CM, Muschler JL, Veiseh M, Vonderhaar BK, Bissell MJ. Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. J Cell Biol. 2009;184:57–66.
Xu R, Spencer VA, Bissell MJ. Extracellular matrix-regulated gene expression requires cooperation of SWI/SNF and transcription factors. J Biol Chem. 2007;282:14992–9.
Rosen JM, Wyszomierski SL, Hadsell D. Regulation of milk protein gene expression. Annu Rev Nutr. 1999;19:407–36.
Wyszomierski SL, Rosen JM. Cooperative effects of STAT5 (signal transducer and activator of transcription 5) and C/EBPbeta (CCAAT/enhancer-binding protein-beta) on beta-casein gene transcription are mediated by the glucocorticoid receptor. Mol Endocrinol. 2001;15:228–40.
Doppler W, Welte T, Philipp S. CCAAT/enhancer-binding protein isoforms beta and delta are expressed in mammary epithelial cells and bind to multiple sites in the beta-casein gene promoter. J Biol Chem. 1995;270:17962–9.
Raught B, Liao S-L, Rosen JM. Developmentally and hormonally regulated CCAAT/enhancer-binding protein isoforms influence beta-casein genee expression. Mol Endocrinol. 1995;9:1223–32.
Inman CK, Li N, Shore P. Oct-1 counteracts autoinhibition of Runx2 DNA binding to form a novel Runx2/Oct-1 complex on the promoter of the mammary gland-specific gene beta-casein. Mol Cell Biol. 2005;25:3182–93.
Dong B, Zhao FQ. Involvement of the ubiquitous Oct-1 transcription factor in hormonal induction of beta-casein gene expression. Biochem J. 2007;401:57–64.
Zhao FQ, Adachi K, Oka T. Involvement of Oct-1 in transcriptional regulation of beta-casein gene expression in mouse mammary gland. Biochim Biophys Acta. 2002;1577:27–37.
Astrand C, Belikov S, Wrange O. Histone acetylation characterizes chromatin presetting by NF1 and Oct1 and enhances glucocorticoid receptor binding to the MMTV promoter. Exp Cell Res. 2009;315:2604–15.
Murayama A, Sakura K, Nakama M, et al. A specific CpG site demethylation in the human interleukin 2 gene promoter is an epigenetic memory. EMBO J. 2006;25:1081–92.
Ning YM, Robins DM. AML3/CBFalpha1 is required for androgen-specific activation of the enhancer of the mouse sex-limited protein (Slp) gene. J Biol Chem. 1999;274:30624–30.
Prefontaine GG, Walther R, Giffin W, Lemieux ME, Pope L, Hache RJ. Selective binding of steroid hormone receptors to octamer transcription factors determines transcriptional synergism at the mouse mammary tumor virus promoter. J Biol Chem. 1999;274:26713–9.
Brockman JL, Schuler LA. Prolactin signals via Stat5 and Oct-1 to the proximal cyclin D1 promoter. Mol Cell Endocrinol. 2005;239:45–53.
Magne S, Caron S, Charon M, Rouyez MC, Dusanter-Fourt I. STAT5 and Oct-1 form a stable complex that modulates cyclin D1 expression. Mol Cell Biol. 2003;23:8934–45.
Kim S, Koga T, Isobe M, et al. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev. 2003;17:1979–91.
Westendorf JJ. Transcriptional co-repressors of Runx2. J Cell Biochem. 2006;98:54–64.
Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat Rev Mol Cell Biol. 2009;10:526–37.
Schuettengruber B, Cavalli G. Recruitment of polycomb group complexes and their role in the dynamic regulation of cell fate choice. Development. 2009;136:3531–42.
Trowbridge JJ, Snow JW, Kim J, Orkin SH. DNA methyltransferase 1 is essential for and uniquely regulates hematopoietic stem and progenitor cells. Cell Stem Cell. 2009;5:442–9.
Fan G, Beard C, Chen RZ, et al. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J Neurosci. 2001;21:788–97.
Lim DA, Huang YC, Swigut T, et al. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–33.
Bloushtain-Qimron N, Yao J, Snyder EL, et al. Cell type-specific DNA methylation patterns in the human breast. Proc Natl Acad Sci U S A. 2008;105:14076–81.
Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–25.
LaMarca HL, Rosen JM. Minireview: hormones and mammary cell fate–what will I become when I grow up? Endocrinology. 2008;149:4317–21.
Pietersen AM, Evers B, Prasad AA, et al. Bmi1 regulates stem cells and proliferation and differentiation of committed cells in mammary epithelium. Curr Biol. 2008;18:1094–9.
Bouras T, Pal B, Vaillant F, et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–41.
Buono KD, Robinson GW, Martin C, et al. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006;293:565–80.
Boras-Granic K, Wysolmerski JJ. Wnt signaling in breast organogenesis. Organogenesis. 2008;4:116–22.
Badders NM, Goel S, Clark RJ, et al. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One. 2009;4:e6594.
Lindvall C, Zylstra CR, Evans N, et al. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One. 2009;4:e5813.
Li W, Ferguson BJ, Khaled WT, et al. PML depletion disrupts normal mammary gland development and skews the composition of the mammary luminal cell progenitor pool. Proc Natl Acad Sci U S A. 2009;106:4725–30.
Gu B, Sun P, Yuan Y, et al. Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation. J Cell Biol. 2009;185:811–26.
Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–9.
Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–55.
Oakes SR, Naylor MJ, Asselin-Labat ML, et al. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes Dev. 2008;22:581–6.
Yamaji D, Na R, Feuermann Y, et al. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 2009;23:2382–7.
Kurpios NA, MacNeil L, Shepherd TG, Gludish DW, Giacomelli AO, Hassell JA. The Pea3 Ets transcription factor regulates differentiation of multipotent progenitor cells during mammary gland development. Dev Biol. 2009;325:106–21.
Fernandez-Valdivia R, Mukherjee A, Ying Y, et al. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol. 2009;328:127–39.
Booth BW, Boulanger CA, Anderson LH, Jimenez-Rojo L, Brisken C, Smith GH. Amphiregulin mediates self-renewal in an immortal mammary epithelial cell line with stem cell characteristics. Exp Cell Res. 2009.
Ng RK, Dean W, Dawson C, et al. Epigenetic restriction of embryonic cell lineage fate by methylation of Elf5. Nat Cell Biol. 2008;10:1280–90.
Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26.
Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–76.
Lelievre SA. Contributions of extracellular matrix signaling and tissue architecture to nuclear mechanisms and spatial organization of gene expression control. Biochim Biophys Acta. 2009;1790:925–35.
Le Beyec J, Xu R, Lee SY, et al. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res. 2007;313:3066–75.
Benton G, Crooke E, George J. Laminin-1 induces E-cadherin expression in 3-dimensional cultured breast cancer cells by inhibiting DNA methyltransferase 1 and reversing promoter methylation status. FASEB J. 2009;23:3884–95.
Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ. Division of labor among the alpha6beta4 integrin, beta1 integrins, and an E3 laminin receptor to signal morphogenesis and beta-casein expression in mammary epithelial cells. Mol Biol Cell. 1999;10:2817–28.
Yang X, Pursell B, Lu S, Chang TK, Mercurio AM. Regulation of beta 4-integrin expression by epigenetic modifications in the mammary gland and during the epithelial-to-mesenchymal transition. J Cell Sci. 2009;122:2473–80.
Kyle UG, Pichard C. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care. 2006;9:388–94.
Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–9.
De Assis S, Hilakivi-Clarke L. Timing of dietary estrogenic exposures and breast cancer risk. Ann N Y Acad Sci. 2006;1089:14–35.
Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17:340–8.
Soto AM, Vandenberg LN, Maffini MV, Sonnenschein C. Does breast cancer start in the womb? Basic Clin Pharmacol Toxicol. 2008;102:125–33.
Fernandez-Twinn DS, Ekizoglou S, Gusterson BA, Luan J, Ozanne SE. Compensatory mammary growth following protein restriction during pregnancy and lactation increases early-onset mammary tumor incidence in rats. Carcinogenesis. 2007;28:545–52.
Burdge GC, Lillycrop KA, Jackson AA. Nutrition in early life, and risk of cancer and metabolic disease: alternative endings in an epigenetic tale? Br J Nutr. 2009;101:619–30.
Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18:2627–38.
Thomas MJ, Seto E. Unlocking the mechanisms of transcription factor YY1: are chromatin modifying enzymes the key? Gene. 1999;236:197–208.
Meier VS, Groner B. The nuclear factor YY1 participates in repression of the beta-casein gene promoter in mammary epithelial cells and is counteracted by mammary gland factor during lactogenic hormone induction. Mol Cell Biol. 1994;14:128–37.
Raught B, Khursheed B, Kazansky A, Rosen J. YY1 represses beta-casein gene expression by preventing the formation of a lactation-associated complex. Mol Cell Biol. 1994;14:1752–63.
Rosen JM, Zahnow C, Kazansky A, Raught B. Composite response elements mediate hormonal and developmental regulation of milk protein gene expression. Biochem Soc Symp. 1998;63:101–13.
Herranz N, Pasini D, Diaz VM, et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol. 2008;28:4772–81.
Kishimoto M, Fujiki R, Takezawa S, et al. Nuclear receptor mediated gene regulation through chromatin remodeling and histone modifications. Endocr J. 2006;53:157–72.
Li X, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol. 2003;23:3763–73.
Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–8.
Amaral PP, Mattick JS. Noncoding RNA in development. Mamm Genome. 2008;19:454–92.
Barski A, Jothi R, Cuddapah S, et al. Chromatin poises miRNA- and protein-coding genes for expression. Genome Res. 2009;19:1742–51.
Mayer C, Schmitz KM, Li J, Grummt I, Santoro R. Intergenic transcripts regulate the epigenetic state of rRNA genes. Mol Cell. 2006;22:351–61.
Guil S, Esteller M. DNA methylomes, histone codes and miRNAs: tying it all together. Int J Biochem Cell Biol. 2009;41:87–95.
Liu CG, Calin GA, Meloon B, et al. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci U S A. 2004;101:9740–4.
Avril-Sassen S, Goldstein LD, Stingl J, et al. Characterisation of microRNA expression in post-natal mouse mammary gland development. BMC Genomics. 2009;10:548.
Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007;21:3238–43.
Sdassi N, Silveri L, Laubier J, et al. Identification and characterization of new miRNAs cloned from normal mouse mammary gland. BMC Genomics. 2009;10:149.
Silveri L, Tilly G, Vilotte JL, Le Provost F. MicroRNA involvement in mammary gland development and breast cancer. Reprod Nutr Dev. 2006;46:549–56.
Wang C, Li Q. Identification of differentially expressed microRNAs during the development of Chinese murine mammary gland. J Genet Genomics. 2007;34:966–73.
Gu Z, Eleswarapu S, Jiang H. Identification and characterization of microRNAs from the bovine adipose tissue and mammary gland. FEBS Lett. 2007;581:981–8.
Shimono Y, Zabala M, Cho RW, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603.
Tanaka T, Haneda S, Imakawa K, Sakai S, Nagaoka K. A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation. 2009;77:181–7.
Greene SB, Gunaratne PH, Hammond SH, Rosen JM. A putative role for microRNA-205 in progenitors of mammary epithelial cells. J Cell Sci. 2010; in press.
Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008;105:1608–13.
Li LC, Okino ST, Zhao H, et al. Small dsRNAs induce transcriptional activation in human cells. Proc Natl Acad Sci U S A. 2006;103:17337–42.
Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–23.
Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–6.
Nagano T, Mitchell JA, Sanz LA, et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–20.
Pandey RR, Mondal T, Mohammad F, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32:232–46.
Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–72.
Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7.
Ginger MR, Shore AN, Contreras A, et al. A noncoding RNA is a potential marker of cell fate during mammary gland development. Proc Natl Acad Sci U S A. 2006;103:5781–6.
Bloushtain-Qimron N, Yao J, Shipitsin M, Maruyama R, Polyak K. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–17.
Dahl JA, Collas P. MicroChIP-a rapid micro chromatin immunoprecipitation assay for small cell samples and biopsies. Nucleic Acids Res. 2008;36:e15.
Goren A, Ozsolak F, Shoresh N, et al. Chromatin profiling by directly sequencing small quantities of immunoprecipitated DNA. Nat Methods. 2009;7:47–9.
Elnitski L, Riemer C, Burhans R, Hardison R, Miller W. MultiPipMaker: comparative alignment server for multiple DNA sequences. Curr Protoc Bioinformatics. 2005;Chapter 10:Unit10 14.
Wen B, Wu H, Shinkai Y, Irizarry RA, Feinberg AP. Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells. Nat Genet. 2009;41:246–50.
Acknowledgement of financial support
USDA/ARS 6250-51000-048-00, NIH 1R21HD053762, and NIH 5R03HD56090 to MR; NIH R37-CA16303-35 to JMR; Iranian Ministry of Science, Research and Technology to MBMT and INRA-292 and P00258 to ED
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rijnkels, M., Kabotyanski, E., Montazer-Torbati, M.B. et al. The Epigenetic Landscape of Mammary Gland Development and Functional Differentiation. J Mammary Gland Biol Neoplasia 15, 85–100 (2010). https://doi.org/10.1007/s10911-010-9170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-010-9170-4