Abstract
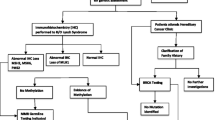
Referral of patients with endometrial (EC) and/or ovarian cancer (OC) for genetic counseling is based on age at diagnosis and family history. Many patients with hereditary cancers are missed by following this strategy. We determined acceptance and mutation detection rate of offering genetic counseling and testing to unselected EC and OC patients. Therefore, in 2007, EC and OC patients were invited for genetic counseling and testing. Patients were asked for their reasons to accept or decline. Nineteen out of fifty-two EC patients (36 %) and twenty-two out of thirty-five OC patients (63 %) accepted genetic counseling, mainly to receive risk assessment for themselves and relatives. Counseling was declined mainly because patients did not want more tests or had no relatives for whom it was relevant. Eighteen out of nineteen EC patients (95 %) and twenty out of twenty-two OC patients (91 %) underwent genetic testing. One EC patient carried an MSH6 mutation (mutation detection rate: 6 %). BRCA1/2 mutations were found in two out of twenty OC patients (10 %). Eleven patients (29 %) received surveillance recommendations for themselves and their relatives. Finally, family history recorded by the gynecologist was compared to that taken by the clinical geneticist. Gynecologists reported family history in ten out of forty-one participants (24 %). In conclusion, genetic counseling and testing are acceptable to patients with OC and/or EC. The 10 % BRCA1/2 mutation detection rate and underreporting of family history by gynecologists warrant referral for genetic counseling for all OC patients, followed by BRCA1/2 testing if indicated. We recommend that microsatellite instability and immunohistochemical analysis be performed in all EC patients, followed by genetic counseling if appropriate. These strategies will lead to better cancer prevention in gynecological cancer patients and their relatives.


Similar content being viewed by others
References
Aarnio, M., Sankila, R., Pukkala, E., Salovaara, R., Aaltonen, L. A., de la Chapelle, A., et al. (1999). Cancer risks in mutation carriers of DNA-mismatch-repair genes. International Journal of Cancer, 81(2), 214–218.
American Society of Clinical Oncology. (2003). Statement of the American Society of Clinical Oncology policy statement update: Genetic Testing for Cancer susceptibility. Journal of Clinical Oncology, 21(12), 2397–2406.
Armstrong, K., Calzone, K., Stopfer, J., Fitzgerald, G., Coyne, J., & Weber, B. (2000). Factors associated with decisions about clinical BRCA1/2 testing. Cancer Epidemiology, Biomarkers and Prevention, 9(11), 1251–1254.
Auranen, A., & Joutsiniemi, T. (2011). A systematic review of gynecological cancer surveillance in women belonging to hereditary nonpolyposis colorectal cancer (Lynch syndrome) families. ACTA Obstetricia et Gynecologica Scandinavica, 90(5), 437–444.
Backes, F. J., Hampel, H., Backes, K. A., Vaccarello, L., Lewandowski, G., Bell, J. A., et al. (2009). Are prediction models for Lynch syndrome valid for probands with endometrial cancer? Familial Cancer, 8(4), 483–487.
Backes, F. J., Mitchell, E., Hampel, H., & Cohn, D. E. (2011). Endometrial cancer patients and compliance with genetic counseling: room for improvement. Gynecologic Oncology, 123(3), 532–536.
Baer, H. J., Brawarsky, P., Murray, M. F., & Haas, J. S. (2010). Familial risk of cancer and knowledge and use of genetic testing. Journal of General Internal Medicine, 25(7), 717–724.
Balmaña, J., Diez, O., Rubio, I., Castiglione, M., & ESMO Guidelines Working Group. (2010). BRCA in breast cancer: ESMO Clinical Practice Guidelines. Annals of Oncology, 21(Suppl 5), v20–22.
Banno, K., Susumu, N., Yanokura, M., Hirao, T., Iwata, T., Hirasawa, A., et al. (2004). Association of HNPCC and endometrial cancers. International Journal of Clinical Oncology, 9(4), 262–269.
Bonadona, V., Bonaïti, B., Olschwang, S., Grandjouan, S., Huiart, L., Longy, M., et al. (2011). Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. Journal of the American Medical Association, 305(22), 2304–2310.
Brozek, I., Ochman, K., Debniak, J., Morzuch, L., Ratajska, M., Stepnowska, M., et al. (2008). High frequency of BRCA1/2 germline mutations in consecutive ovarian cancer patients in Poland. Gynecologic Oncology, 108(2), 433–437.
Devlin, L., Graham, C. A., Price, J. H., & Morrison, P. J. (2008). Germline MSH6 mutations are more prevalent in endometrial cancer patient cohorts than hereditary non polyposis colorectal cancer cohorts. Ulster Medical Journal, 77(1), 25–30.
Domanska, K., Carlsson, C., Bendahl, P. O., & Nilbert, M. (2009). Knowledge about hereditary non-polyposis colorectal cancer; mutation carriers and physicians at equal levels. BMC Medical Genetics, 10, 30.
Dove-Edwin, I., Sasieni, P., Adams, J., & Thomas, H. J. (2005). Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. British Medical Journal, 331(7524), 1047.
Drew, Y., & Calvert, H. (2008). The potential of PARP inhibitors in genetic breast and ovarian cancers. Annals of the New York Academy of Sciences, 1138, 136–145.
Dutch Society for Clinical Genetics (VKGN). (2008). Guideline hereditary colorectal cancer. Oisterwijk: Van den Boogaard Print- &Mediamanagement. http://www.oncoline.nl.
Dutch Society for Clinical Genetics (VKGN). (2012). Guideline breast cancer 2012. http://www.oncoline.nl.
Frank, T. S., Deffenbaugh, A. M., Reid, J. E., Hulick, M., Ward, B. E., Lingenfelter, B., et al. (2002). Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. Journal of Clinical Oncology, 20(6), 1480–1490.
Garg, K., & Soslow, R. A. (2009). Lynch syndrome (hereditary non-polyposis colorectal cancer) and endometrial carcinoma. Journal of Clinical Pathology, 62(8), 679–684.
Geer, K. P., Ropka, M. E., Cohn, W. F., Jones, S. M., & Miesfeldt, S. (2001). Factors influencing patients’ decisions to decline cancer genetic counseling services. Journal of Genetic Counseling, 10(1), 25–40.
Godard, B., Pratte, A., Dumont, M., Simard-Lebrun, A., & Simard, J. (2007). Factors associated with an individual’s decision to withdraw from genetic testing for breast and ovarian cancer susceptibility: implications for counseling. Genetic Testing, 11(1), 45–54.
Goodfellow, P. J., Buttin, B. M., Herzog, T. J., Rader, J. S., Gibb, R. K., Swisher, E., et al. (2003). Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proceedings of the National Academy of the Sciences, 100(10), 5908–5913.
Hampel, H., Frankel, W., Panescu, J., Lockman, J., Sotamaa, K., Fix, D., et al. (2006). Screening for Lynch syndrome (Hereditary Nonpolyposis Colorectal Cancer) among endometrial cancer patients. Cancer Research, 66(15), 7810–7817.
Hes, F. J. (2008). Lynch syndrome: still not a familiar picture. World Journal of Surgical Oncology, 6, 21.
Hoogerbrugge, N. (2010). Recognizing hereditary cancer: new simple referral criteria for solid cancers. Netherlands Journal of Oncology, 7, 330–337.
Jacobi, C. E., Van Ierland, Y., Van Asperen, C. J., Hallensleben, E., Devilee, P., et al. (2007). Prediction of BRCA1/2 mutation status in patients with ovarian cancer from a hospital-based cohort. Genetics in Medicine, 9(3), 173–179.
Järvinen, H. J., Aarnio, M., Mustonen, H., Aktan-Collan, K., Aaltonen, L. A., Peltomäki, P., et al. (2000). Controlled 15-year trial on screening for colorectal cancer in families with hereditary non-polyposis colorectal cancer. Gastroenterology, 118(5), 829–834.
Julian-Reynier, C., Eisinger, F., Chabal, F., Aurran, Y., Bignon, Y. J., Noguès, C., et al. (1998). Cancer genetic clinics: why do women who already have cancer attend? European Journal of Cancer, 34(10), 1549–1553.
Khoo, U. S., Ngan, H. Y., Cheung, A. N., Chan, K. Y., Lu, J., Chan, V. W., et al. (2000). Mutational analysis of BRCA1 and BRCA2 genes in Chinese ovarian cancer identifies 6 novel germline mutations. Human Mutation, 16(1), 88–89.
Khoo, U. S., Chan, K. Y., Cheung, A. N., Xue, W. C., Shen, D. H., Fung, K. Y., et al. (2002). Recurrent BRCA1 and BRCA2 germline mutations in ovarian cancer: a founder mutation of BRCA1 identified in the Chinese population. Human Mutation, 19(3), 307–308.
Lacour, R. A., Daniels, M. S., Westin, S. N., Meyer, L. A., Burke, C. C., Burns, K. A., et al. (2008). What women with ovarian cancer think and know about genetic testing. Gynecologic Oncology, 111(1), 132–136.
Leenen, C. H., Van Lier, M. G., Van Doorn, H. C., Van Leerdam, M. E., Kooi, S. G., De Waard, J., et al. (2012). Prospective evaluation of molecular screening for Lynch syndrome in patients with endometrial cancer ≤ 70 years. Gynecologic Oncology, 125(2), 414–420.
Liede, A., Malik, I. A., Aziz, Z., De los Rios, P., Kwan, E., & Narod, S. A. (2002). Contribution of BRCA1 and BRCA2 mutations to breast and ovarian cancer in Pakistan. American Journal of Human Genetics, 71(3), 595–606.
Ligtenberg, M. J., Kuiper, R. P., Chan, T. L., Goossens, M., Hebeda, K. M., Voorendt, M., et al. (2009). Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3' exons of TACSTD1. Nature Genetics, 41(1), 112–117.
Lim, M. C., Seo, S. S., Kang, S., Seong, M. W., Lee, B. Y., & Park, S. Y. (2010). Hereditary non-polyposis colorectal cancer/Lynch syndrome in Korean patients with endometrial cancer. Japanese Journal of Clinical Oncology, 40(12), 1121–1127.
Long, K. C., & Kauff, N. D. (2011). Hereditary ovarian cancer: recent molecular insights and their impact on screening strategies. Current Opinion in Oncology, 23(5), 526–530.
Lynch, H. T., Watson, P., Tinley, S., Snyder, C., Durham, C., Lynch, J., et al. (1999). An update on DNA-based BRCA1/BRCA2 genetic counseling in hereditary breast cancer. Cancer Genetica and Cytogenetics, 109(2), 91–98.
Majdak, E. J., De Bock, G. H., Brozek, I., Perkowska, M., Ochman, K., Debniak, J., et al. (2005). Prevalence and clinical correlations of BRCA1/BRCA2 unclassified variant carriers among unselected primary ovarian cancer cases – preliminary report. European Journal of Cancer, 41(1), 143–150.
Malander, S., Ridderheim, M., Måsbäck, A., Loman, N., Kristoffersson, U., Olsson, H., et al. (2004). One in 10 ovarian cancer patients carry germ line BRCA1 or BRCA2 mutations: results of a prospective study in Southern Sweden. European Journal of Cancer, 40(3), 422–428.
Manders, P., Spruijt, L., Kets, C. M., Willems, H. W., Bodmer, D., Hebeda, K. M., et al. (2011). Young age and a positive family history of colorectal cancer are complementary selection criteria for the identification of Lynch syndrome. European Journal of Cancer, 47(9), 1407–1413.
Meyer, L. A., Anderson, M. E., Lacour, R. A., Suri, A., Daniels, M. S., Urbauer, D. L., et al. (2010). Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: missed opportunities. Obstetrics and Gynecology, 115(5), 945–952.
Murphy, M. A., & Wentzensen, N. (2011). Frequency of mismatch repair deficiency in ovarian cancer: a systematic review. International Journal of Cancer, 129, 1914–1922.
Netherlands Foundation for the Detection of Hereditary Tumours (STOET) and Dutch Society for Clinical Genetics (VKGN). (2005, 2010). Hereditary Tumours: guidelines for diagnostics and prevention. 3rd edition, 2005 and 4th edition
Pal, T., Permuth-Wey, J., Betts, J. A., Krischer, J. P., Fiorica, J., Arango, H., et al. (2005). BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer, 104(12), 2807–2816.
Plaschke, J., Engel, C., Krüger, S., Holinski-Feder, E., Pagenstecher, C., Mangold, E., et al. (2004). Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: the German hereditary nonpolyposis colorectal cancer consortium. Journal of Clinical Oncology, 22(22), 4486–4494.
Pruthi, S., Gostout, B. S., & Lindor, N. M. (2010). Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clinic Proceedings, 85(12), 1111–1120.
Ramsoekh, D., Wagner, A., Van Leerdam, M. E., Dinjens, W. N., Steyerberg, E. W., Halley, D. J., et al. (2008). A high incidence of MSH6 mutations in Amsterdam criteria II-negative families tested in a diagnostic setting. Gut, 57(11), 1539–1544.
Ready, K. J., Daniels, M. S., Sun, C. C., Peterson, S. K., Northrup, H., & Lu, K. H. (2010). Obstetrics/gynecology residents’ knowledge of hereditary breast and ovarian cancer and Lynch syndrome. Journal of Cancer Education, 25(3), 401–404.
Renkonen-Sinisalo, L., Bützow, R., Leminen, A., Lehtovirta, P., Mecklin, J. P., & Järvinen, H. J. (2006). Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. International Journal of Cancer, 120(4), 821–824.
Resnick, K., Straughn, J. M., Jr., Backes, F., Hampel, H., Matthews, K. S., & Cohn, D. E. (2009). Lynch syndrome screening strategies among newly diagnosed endometrial cancer patients. Obstetrics and Gynecology, 114(3), 530–536.
Risch, H. A., McLaughlin, J. R., Cole, D. E., Rosen, B., Bradley, L., Fan, I., et al. (2006). Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. Journal of the National Cancer Institute, 98(23), 1694–1706.
Rubin, S. C., Blackwood, M. A., Bandera, C., Behbakht, K., Benjamin, I., et al. (1998). BRCA1, BRCA2, and hereditary nonpolyposis colorectal cancer gene mutations in an unselected ovarian cancer population: relationship to family history and implications for genetic testing. American Journal of Obstetrics and Gynecology, 178(4), 670–677.
Sarantaus, L., Vahteristo, P., Bloom, E., Tamminen, A., Unkila-Kallio, L., et al. (2001). BRCA1 and BRCA2 mutations among 233 unselected Finnish ovarian carcinoma patients. European Journal of Human Genetics, 9(6), 424–430.
Schlich-Bakker, K. J., Ten Kroode, H. F., Wárlám-Rodenhuis, C. C., Van den Bout, J., & Ausems, M. G. (2007). Barriers to participating in genetic counseling and BRCA testing during primary treatment for breast cancer. Genetics in Medicine, 9(11), 766–777.
Sijmons, R. H., Boonstra, A. E., Reefhuis, J., Hordijk-Hos, J. M., De Walle, H. E., et al. (2000). Accuracy of family history of cancer: clinical genetic implications. European Journal of Human Genetics, 8(3), 181–186.
Soegaard, M., Kjaer, S. K., Cox, M., Wozniak, E., Høgdall, E., Høgdall, C., et al. (2008). BRCA1 and BRCA2 mutation prevalence and clinical characteristics of a population-based series of ovarian cancer cases from Denmark. Clinical Cancer Research, 14(12), 3761–3767.
Netherlands Cancer Registry and Statistics 2008. http://www.iknl.nl.
Tonin, P. N., Mes-Masson, A. M., Narod, S. A., Ghadirian, P., & Provencher, D. (1999). Founder BRCA1 and BRCA2 mutations in French Canadian ovarian cancer cases unselected for family history. Clinical Genetics, 55(5), 318–324.
Tonin, P. N., Maugard, C. M., Perret, C., Mes-Masson, A. M., & Provencher, D. M. (2007). A review of histopathological subtypes of ovarian cancer in BRCA-related French Canadian cancer families. Familial Cancer, 6(4), 491–497.
Trainer, A. H., Meiser, B., Watts, K., Mitchell, G., Tucker, K., & Friedlander, M. (2010). Moving toward personalized medicine: treatment-focused genetic testing of women newly diagnosed with ovarian cancer. International Journal of Gynecological Cancer, 20(5), 704–716.
Umar, A., Boland, C. R., Terdiman, J. P., Syngal, S., De la Chapelle, A., Rüschoff, J., et al. (2004). Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. Journal of the National Cancer Institute, 96(4), 261–268.
Van Asperen, C. J., Van Dijk, S., Zoeteweij, M. W., Timmermans, D. R., De Bock, G. H., Meijers-Heijboer, E. J., et al. (2002). What do women really want to know? Motives for attending familial breast cancer clinics. Journal of Medical Genetics, 39(6), 410–414.
Van der Kolk, D. M., de Bock, G. H., Leegte, B. K., Schaapveld, M., Mourits, M. J., de Vries, J., et al. (2010). Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Research and Treatment, 124(3), 643–651.
Van Riel, E., Wárlám-Rodenhuis, C. C., Verhoef, S., Rutgers, E. J., & Ausems, M. G. (2010). BRCA testing of breast cancer patients: medical specialists’ referral patterns, knowledge and attitudes to genetic testing. European Journal of Cancer Care, 19(3), 369–376.
Vasen, H. F. (2005). Clinical description of the Lynch syndrome [hereditary nonpolyposis colorectal cancer (HNPCC)]. Familial Cancer, 4(3), 219–225.
Vasen, H. F., Watson, P., Mecklin, J. P., & Lynch, H. T. (1999). New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch Syndrome) proposed by the international collaborative group on HNPCC. Gastroenterology, 116(6), 1453–1456.
Weissman, S. M., Burt, R., Church, J., Erdman, S., Hampel, H., Holter, S., et al. (2012). Identification of individuals at risk for lynch syndrome using targeted evaluations and genetic testing: national society of genetic counselors and the collaborative group of the Americas on inherited colorectal cancer joint practice guideline. Journal of Genetic Counseling, 21(4), 484–493.
Wevers, M. R., Hahn, D. E., Verhoef, S., Bolhaar, M. D., Ausems, M. G., Aaronson, N. K., et al. (2012). Breast cancer genetic counseling after diagnosis but before treatment: A pilot study on treatment consequences and psychological impact. Patient Education and Counseling; in press
Wideroff, L., Vadaparampil, S. T., Greene, M. H., Taplin, S., Olson, L., & Freedman, A. N. (2005). Hereditary breast/ovarian and colorectal cancer genetics knowledge in a national sample of US physicians. Journal of Medical Genetics, 42(10), 749–755.
Wijnen, J., De Leeuw, W., Vasen, H., Van der Klift, H., Møller, P., Stormorken, A., et al. (1999). Familial endometrial cancer in female carriers of MSH6 germline mutations. Nature Genetics, 23(2), 142–144.
Yazici, H., Glendon, G., Yazici, H., Burnie, S. J., Saip, P., Buyru, F., et al. (2002). BRCA1 and BRCA2 mutations in Turkish familial and non-familial ovarian cancer patients: a high incidence of mutations in non-familial cases. Human Mutation, 20(1), 28–34.
Yong, M. C., Zhou, X. J., & Lee, S. C. (2003). The importance of paternal family history in hereditary breast cancer is underappreciated by health care professionals. Oncology, 64(3), 220–226.
Zilliacus, E., Meiser, B., Gleeson, M., Watts, K., Tucker, K., Lobb, E. A., et al. (2012) Are we being overly cautious? A qualitative inquiry into the experiences and perceptions of treatment-focused germline BRCA genetic testing amongst women recently diagnosed with breast cancer. Supportive Care in Cancer; in press.
Acknowledgements
We thank E. van Riel and A. Schoemaker, genetic counselors, for their help in counseling the patients, and E. Hennekam for tracking the patient data in the Dutch Civil Register. A.Y. Bronkhorst, W. Gaasbeek, R.P.M. Jansen and P.H.A. van Zon (Section of Genome Diagnostics, Department of Medical Genetics, University Medical Center Utrecht) are thanked for performing molecular analysis of the BRCA1/2 genes in the patients. Also, we would like to thank N. Hoogerbrugge and W. van Zelst-Stams for their helpful suggestions with the interpretation of the results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dekker, N., van Dorst, E.B.L., van der Luijt, R.B. et al. Acceptance of Genetic Counseling and Testing in a Hospital-Based Series of Patients with Gynecological Cancer. J Genet Counsel 22, 345–357 (2013). https://doi.org/10.1007/s10897-012-9553-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-012-9553-3