Abstract
Although many lake restoration projects have led to decreased nutrient loads and increased water transparency, the establishment or expansion of macrophytes does not immediately follow the improved abiotic conditions and it is often unclear whether vegetation with high macrophyte diversity will return. We provide an overview of the potential bottlenecks for restoration of submerged macrophyte vegetation with a high biodiversity and focus on the biotic factors, including the availability of propagules, herbivory, plant competition and the role of remnant populations. We found that the potential for restoration in many lakes is large when clear water conditions are met, even though the macrophyte community composition of the early 1900s, the start of human-induced large-scale eutrophication in Northwestern Europe, could not be restored. However, emerging charophytes and species rich vegetation are often lost due to competition with eutrophic species. Disturbances such as herbivory can limit dominance by eutrophic species and improve macrophyte diversity. We conclude that it is imperative to study the role of propagule availability more closely as well as the biotic interactions including herbivory and plant competition. After abiotic conditions are met, these will further determine macrophyte diversity and define what exactly can be restored and what not.
Similar content being viewed by others
Introduction
Macrophytes play an important structuring role in shallow freshwater bodies (Scheffer et al., 2001; Burks et al., 2006). Macrophytes have traits that affect the ecosystem services that shallow water bodies provide as they can maintain clear water and nutrient retention, while they also strongly improve aquatic biodiversity by providing a habitat and food for many aquatic organisms (Carpenter & Lodge, 1986). The ongoing eutrophication of freshwater bodies (Carpenter et al., 1998; Tilman et al., 2001) has induced a decline or disappearance of macrophytes from many shallow water ecosystems (Sand-Jensen et al., 2000; Brouwer & Roelofs, 2001; Gulati & van Donk, 2002; Lamers et al., 2002). This has been observed in many shallow lakes in densely populated areas, for instance in the Loosdrecht lakes (Best et al., 1984; Gulati & van Donk, 2002; Van de Haterd & Ter Heerdt, 2007) and Lake Veluwemeer (Van den Berg et al., 1999; Ibelings et al., 2007) in The Netherlands, Lake Fure (Sand-Jensen et al., 2008) and Lake Arresø (Jeppesen et al., 2007) in Denmark and the Müggelsee in Germany (Korner, 2001). Increased nutrient availability can initially stimulate macrophyte growth as long as the water remains clear (Lombardo & Cooke, 2003; Nagasaka, 2004; Feuchtmayr et al., 2009). However, with increasing nutrient loading, phytoplankton biomass may increase, creating water turbidity which may result in light limitation and disappearance of submerged macrophytes (Scheffer et al., 1993). However, before the water becomes turbid, there can be direct shading of macrophyte leaves by the accumulation of epiphyton or filamentous algae, which causes macrophyte decline or inhibits their return (Phillips et al., 1978; Weisner et al., 1997; Jones & Sayer, 2003; Roberts et al., 2003; Irfanullah & Moss, 2004; Hilt et al., 2010). Besides the indirect effect of nutrients on macrophyte growth (via light limitation), certain nutrients can be toxic for macrophytes, including ammonium which can be toxic at high concentrations for many macrophyte species (Smolders & Roelofs, 1996), whereas nitrate has been shown to reduce the growth of Chara species (Lambert & Davy, 2011). Furthermore, sulphide, which is formed at high sulphate concentrations in the water or sediment, can be toxic for macrophytes (Van der Welle et al., 2006). Nutrient addition may also induce changes in the fish community which may lead to increased turbidity due to the predation on zooplankton by planktivorous fish or sediment resuspension by benthic feeders (Jeppesen et al., 1997; Gulati & van Donk, 2002). Due to a shift from clear to turbid water with increasing eutrophication, shallow water bodies may eventually become dominated by algae, many species of which can occur in heavy blooms, especially cyanobacteria of certain toxic strains. This has jeopardised several of the important services of shallow waters, including use for drinking water and recreational activities such as swimming (Guo, 2007). To restore ecosystem services and aquatic biodiversity, many restoration programmes have been set up to induce backward shifts from the turbid, algal-dominated state to a clear state dominated by macrophytes (Moss, 1989; Scheffer et al., 1993; Jeppesen et al., 2005a, b). As macrophytes play a crucial role in the maintenance of this clear water state, the targets and success of these restoration efforts are often measured in terms of the extent of return of submerged macrophytes. Therefore, most restoration measures try to realise clear water conditions, reasoning that, by restoring clear water conditions, macrophytes will return, which, on their turn, will maintain the clear water state. Restoration measures that can be taken to induce a shift from a turbid to a clear water state have been thoroughly reviewed recently (Gulati & van Donk, 2002; Sondergaard et al., 2007; Gulati et al., 2008; Sondergaard et al., 2008). However, restoring clear water does not always lead to the return of macrophytes or the return of desired species (Lauridsen et al., 2003a; Jeppesen et al., 2005a, b; Sondergaard et al., 2008), nor can macrophytes always maintain the clear water state (Bakker et al., 2010). In this review, we want to pay specific attention to the restoration of macrophyte communities and the factors that determine the biodiversity of this restored vegetation. We limit this review to freshwater submerged macrophytes, including vascular species and charophytes.
We focus on the importance of biotic factors, including the availability of propagules, the amount of herbivory and role of remnant populations, whereas macrophyte requirements for abiotic conditions, such as light and nutrient availability or shelter from the wind are recently reviewed in Bornette & Puijalon (2011). Furthermore, we address the importance of the composition and abundance of the macrophyte vegetation as these may affect the performance of ecosystem functions and conservation value of the vegetation. The study is focused on highlighting potential constraints for the return of a diverse macrophyte vegetation to lakes where abiotic conditions have been restored.
Where do the returning macrophytes come from?
If the right abiotic conditions exist (i.e. mainly enough light, nutrients and shelter), macrophytes can return to a restored shallow water body in the short-term, varying from a few weeks to a few years (Casanova & Brock, 1990; Portielje & Roijackers, 1995; Brouwer et al., 2002; Ter Heerdt & Hootsmans, 2007), although numerous exceptions have been reported (Lamers et al., 2002; Jeppesen et al., 2005a, b; Geurts et al., 2008; Sarneel et al., 2011). Table 1 lists examples of restoration projects where nutrient loading has been reduced or sediment disturbing and zooplanktivorous fish has been removed and the effect on the restoration of the macrophyte community composition. The recovery of the vegetation raises questions about the origin of the returning plants: are propagules already present as a propagule bank or as a remnant population or is there a massive dispersal of macrophyte propagules from other source populations?
Dispersal of propagules
Seeds, oospores and vegetative propagules of submerged macrophytes are most likely dispersed by water, but also by wind and animals (Boedeltje et al., 2002, 2003, Charalambidou & Santamaria, 2005; Soons et al., 2008). In terrestrial ecology, the probability of dispersal via water is quantified by the buoyancy of the seed (Kleyer et al., 2008), assuming that long floating time enhances dispersal. Surprisingly, data on the buoyancy of seeds and other propagules from submerged macrophytes are lacking, but recent studies (Xie et al., 2010) reveal that at least vegetative propagules can float for several months. In shallow lakes, wind plays an important role in the dispersal route as the wind-induced currents transport the seeds (Sarneel, 2010; Soomers et al., 2010). Also, for charophytes, wind dispersal may play a role as spores are very light and generally easily dispersed by the wind. Propagules of aquatic macrophytes are also dispersed by waterfowl, fish and invertebrates (Green et al., 2002; Charalambidou & Santamaria, 2005; Brochet et al., 2010; Figuerola et al., 2010; Pollux, 2011). Especially the smaller-sized propagules are more likely to survive the gut passage in birds feeding on them and germinate afterwards (Soons et al., 2008). After passing through the gut, the frequency of propagule germination for many plants increases, e.g. in Chara spp., Potamogeton pectinatus, P. nodosus, and P. pusillis (Brochet et al., 2010; Figuerola et al., 2010). However, the overall probability of the digested propagules to establish successfully in a new habitat may well be low. Nevertheless, dispersal via animals provides macrophyte species with an opportunity to disperse over long distances, stretching up to 3000 km (Soons et al., 2008). Genetic analyses support the exchange of propagules among distant and upstream populations (Green et al., 2002; Pollux et al., 2009). Therefore, dispersal is a powerful mode for the submerged macrophytes to return to the restored water bodies. However, the undesired species (e.g. eutrophic, very common or invasive species) may often have the highest probability to colonise new sites after restoration, leaving a low probability for colonisation by rare, endangered and desired species. But perhaps, propagules of such target species might already be present in the propagule bank.
The role of the propagule bank
Propagule bank studies of submerged lake sediment are rather scarce, although propagule banks of riparian zones did receive attention. Table 2 shows an overview of the literature available on the presence of macrophytes in both submerged and riparian propagule bank samples. Because the most commonly used seedling emergence test has been developed for terrestrial vegetation, there is no standardisation for sampling the submerged soils: sampling designs vary, with core depth ranging from 2.5 to 26 cm and germination conditions from moist soil to 60 cm flooding. Such large differences will strongly affect the results of the propagule bank assays. Based on trials, Boedeltje et al. (2002) recommend to further standardise aquatic propagule bank research by using moist, but not submerged sediment.
Reported propagule densities range from 0 to 40,000 propagules m−2 for submerged macrophytes (Table 2) indicating that in some cases, macrophytes may not return simply because of a lack of propagules, but in other cases generally high densities ensure their return. The occurrence of propagules of submerged macrophytes is not restricted to the lake bottom sediment but they may also occur in sediment from riparian zones and floodplains. In general, riparian propagule banks have somewhat higher propagule densities compared with propagule banks in lake sediments. From the literature on lake sediments, it is clear that particularly propagules from Chara species can be very abundant (Table 2; De Winton et al., 2000). This may well explain their relatively rapid return in case of many restoration projects (Casanova & Brock, 1990). Other species are less frequently encountered in soil samples, and investigated macrophyte species mainly exhibit transient to short-term persistent propagules (Table 3; Kleyer et al., 2008). Therefore, a lack of propagules may actually inhibit macrophyte return after restoration in some lakes (Strand & Weisner, 2001).
Germination
Aquatic macrophytes may germinate poorly in the field. From the yearly production about 15% of Chara aspera spores germinate (Van den Berg et al., 2001). Recruitment from the dispersed propagules and from the propagule bank may depend on the environmental conditions, including light, soil moisture and nutrient availability (Sederias & Colman, 2007, 2009). Although data on germination of submerged macrophytes are scarce, seedling emergence tests show that propagules of submerged macrophytes can germinate as well on moist and wet sediment (Boedeltje et al., 2002; De Winton, 2000; Espinar & Clemente, 2007) as under water (Harwell & Havens, 2003; Porter et al., 2007). Moreover, P. pectinatus is known to recruit more from seeds with decreasing latitude, due to a higher probability of summer drought at these latitudes, which reduces survival of tubers and thus also their clonal reproduction (Santamaria & Garcia, 2004).
However, many macrophytes do not depend only on recruitment from seeds as they can easily regenerate from fragments. Some even produce specialised vegetative dispersal organs, turions and other vegetative propagules which can regrow easily, even under very low light conditions (Xie et al., 2010). Generally, the clonal recruitment through vegetative propagules is considered to prevail over that from seeds and oospores as they often outnumber seeds in trapping experiments (Boedeltje et al., 2002, 2003). Capers (2003) found that about 60% of the individuals that colonised bare soil in freshwater tidal areas originated from vegetative propagules. Genetic studies, however, show that recruitment from vegetative propagules versus seeds and oospores is very species specific (Nilsson et al., 2010; Bornette & Puijalon, 2011).
The importance of remnant populations
In addition, species can also colonise restored shallow water bodies by expansion of local remnant populations. As most macrophyte species are clonal, theoretically only a single individual needs to survive until favourable conditions return. Generally, the occurrence of macrophyte species shows only a weak relationship with the nutrient concentration in the water (Vestergaard & Sand-Jensen, 2000; Penning et al., 2008; Sondergaard et al., 2010). Possibly, the species temporarily can tolerate the less favourable conditions (Blindow, 1992a; Van den Berg et al., 1999). Based on their long-term dataset (100 years), Sand-Jensen et al. (2008) elegantly show that the return of macrophytes after improved abiotic conditions in Lake Fure in Denmark, was strongly determined by the presence of clones of several species that had originated from the time before eutrophication. The historical presence of clones of species in the lake was a much more powerful predictor of vegetation composition after restoration than the altered nutrient conditions. Thus, in the restoration of shallow water bodies remnant populations, especially for species of high conservation value, deserve special attention and should, if possible, remain unscathed by the restoration measures taken.
On the other hand, the historical presence could also form a threat to successful restoration if undesirable species, e.g. eutrophic or invasive species, are present in the area. These are then also very likely to re-colonise after restoration, especially if restoration measures have not led to an anticipated decrease in the nutrient loading, for example in some cases where biomanipulation is used as a restoration measure (Gulati & Van Donk, 2002; Gulati et al., 2008).
Herbivory on returning macrophytes
When macrophytes return after restoration of shallow water bodies, waterbirds are attracted to this new and abundant food source (Noordhuis et al., 2002). The question is whether grazing by waterfowl and large fish can also prevent or inhibit the re-colonisation of shallow water bodies after restoration? Vertebrate herbivores can strongly reduce macrophyte vegetation, but their impact varies among study sites (Marklund et al., 2002). The question whether herbivores can prevent the colonisation of macrophytes in restored shallow water bodies is debated. Experiments where macrophytes were transplanted in restored lakes showed that herbivores (large fish and waterfowl) strongly reduced macrophyte biomass (Lauridsen et al., 1993; Sondergaard et al., 1996; Lauridsen et al., 2003b; Irfanullah & Moss, 2004; Van de Haterd & Ter Heerdt, 2007; Moore et al., 2010). However, Perrow et al. (1997) and Strand & Weisner (2001) found no significant reduction due to herbivory by fish and birds in restored lakes of the biomass of macrophytes that had developed spontaneously, whereas Hilt (2006) found a more than 90% reduction of P. pectinatus vegetation through grazing. Even if the herbivores do not completely prevent the colonisation of macrophytes, they may retard the vegetation development. As the macrophytes that appear when clear water is restored, are required to maintain this clear water state, a rapid colonisation, i.e. increased coverage by macrophytes of the water body is crucial. If herbivores inhibit the increase in coverage of macrophytes or the biomass that they attain, the colonisation process may become too slow and the clear water phase may disappear, thereby decreasing the probability of macrophyte establishment and dominance (Van de Bund & Van Donk, 2002; Sondergaard et al., 2008). However, in addition to reducing macrophyte biomass, herbivores may also affect macrophyte community composition by selective consumption of certain species in favour of other species. For example, in Lake Zwemlust in the Netherlands, the macrophyte vegetation that had developed after the lake’s restoration by biomanipulation, was markedly grazed down by coots and rudd, shifting the dominance of Elodea nutallii to co-dominance by Ceratophyllum demersum and Potamogeton berchtholdii (Van Donk & Otte, 1996). Waterfowl has been documented to graze selectively on P. pectinatus: in Matsalu bay in Estonia, herbivores selectively removed P. pectinatus plants in favour of the charophytes (Hidding et al., 2010a), whereas in the Lauwersmeer in The Netherlands, waterfowl suppressed dominance of P. pectinatus in favour of subordinate Zannichellia palustris and Potamogeton pusillus (Hidding et al., 2010b).
The role of macrophyte species in ecosystem stability
Macrophytes differ in their efficiency to retain nutrients (Engelhardt & Ritchie, 2001), in their suitability as substrate for macrofauna (McAbendroth et al., 2005; Declerck et al., 2011) and in their importance as food for herbivores (Dorenbosch & Bakker, 2011). Several studies have reported enhanced water clarity above charophyte vegetation (Scheffer et al., 1994; Van Donk & Van de Bund, 2002; Hargeby et al., 2007), although this clearing effect is not limited to charophytes (Kosten et al., 2009b). Charophytes can attain high biomass and form dense stands (Blindow, 1992b; Van Nes et al., 2002; Bakker et al., 2010), which may improve the trapping of sediment. Furthermore, the occurrence of charophyte stands may be more stable than for example those of Potamogeton spp. (Van den Berg et al., 1999) that occur more stochastically. Combined with a strong allelopathic activity of charophytes (Vermaat et al., 2000; Kufel & Kufel, 2002; Mulderij et al., 2003; Hilt & Gross, 2008), clear water conditions may be achieved relatively easily. Waters dominated by eutrophic species such as Potamogeton spp. on the other hand seem to switch more readily to a turbid state (Van Nes et al., 2002). This may be because eutrophic species grow at nutrient-rich conditions which favour algal growth or because of the morphology of these species leading to more biomass allocation towards the water surface and lesser biomass density. Also a switch from a macrophyte community dominated by charophytes to a Potamogeton-dominated vegetation is accompanied by a substantial reduction in the seasonal duration of macrophyte dominance and a greater tendency of incursions by phytoplankton (Sayer et al., 2010). Therefore, the macrophyte community composition seems to affect the ecosystem functions performed by macrophytes. Currently, the importance of the effect of macrophyte community composition remains largely unknown as this is just an emerging topic of research.
Macrophyte biodiversity
The number of species in submerged macrophyte vegetation is generally rather low compared to terrestrial vegetation (Edvardsen & Okland, 2006). Field studies show that macrophyte richness is related to several lake variables, including lake area, altitude, shoreline complexity, connectivity, trophic state, conductivity and water and sediment quality (Rorslett, 1991; Murphy, 2002; Makela et al., 2004; Declerck et al., 2005; Scheffer et al., 2006; Geurts et al., 2008). This makes macrophyte species richness generally hard to predict (Edvardsen & Okland, 2006). However, some general mechanisms and patterns can be acquired from field surveys. As for terrestrial plant species, coexistence in macrophytes is highest at optimal light conditions. Under-water light conditions, which reflect turbidity, are an important limiting factor for macrophyte diversity: for example in fens, macrophytes are restricted to water depths <4 m and to water bodies with a turbidity <20 ppm Pt and for red list species <12 ppm Pt (Geurts, 2010). Shading, including that caused by other macrophytes, may also reduce diversity. Macrophyte species richness follows an optimum curve over a productivity gradient, as earlier described for terrestrial vegetation (Al-Mufti et al., 1977): macrophyte richness peaks at intermediate standing crop, indicating light limitation at high plant production and suboptimal conditions for growth of many species at low productivity due to nutrient limitation (Willby et al., 2001; Murphy, 2002). Therefore, the return of large amounts of macrophytes does not need to coincide with the highest species richness. This is for instance observed in restoration projects in shallow eutrophic lakes, where upon the increase of water transparency, fast-growing eutrophic species such as Elodea nuttallii or C. demersum may initially become dominant, leading to a large coverage of macrophytes, but low species diversity (Hilt et al., 2006). Similarly, the spread of invasive macrophytes can lead to high coverage and large macrophyte biomass, but a low species diversity, as native species may become outcompeted due to shading (Stiers et al., 2011).
Nutrient levels in shallow water bodies do affect macrophyte diversity indirectly through changing light conditions, but also directly through the accumulation of toxic substances. The sediment Fe:PO4 ratios may be used as a diagnostic tool to determine optimal macrophyte diversity. Generally species richness is highest and red list species occur more often at high Fe:PO4 ratios (>10 mol mol−1) because of a higher probability of strong P release, associated with algal blooms and toxic sulphide formation, at ratios below this threshold (Smolders & Roelofs, 1996; Van der Welle et al., 2007; Geurts et al., 2008). The concentration of nitrate in the surface water in winter has also been reported as an important predictor of macrophyte or charophyte species richness in the field (James et al., 2005; Lambert & Davy, 2011); and nitrate loading can also reduce macrophyte species richness under experimental conditions (Barker et al., 2008). This relationship is explained by increased competition at higher nitrate availability resulting in a shift towards floating-leaved macrophyte species and thus light limitation (James et al., 2005) and a toxic effect of nitrate on charophytes (Lambert & Davy, 2011). Macrophyte species richness declined above a threshold concentration of 1–2 mg N-NO3 l−1 in winter (James et al., 2005) or 0.6 mg N-NO3 l−1 (corresponding to 1.5 mg TN l−1) under experimental conditions (Barker et al., 2008). Charophyte species richness declined above a threshold of a mean annual concentration of ca. 2 mg N-NO3 l−1 (Lambert & Davy, 2011).
Even though the role of abiotic factors in determining macrophyte diversity is gradually becoming established, little is yet known about how biotic factors affect macrophyte diversity. It is for example virtually unknown whether the composition of macrophyte vegetation is limited by propagule availability or local conditions, which may affect colonisation, establishment and growth. Analyses of patterns of macrophyte diversity among lakes for examining the role of the regional species pool, by including a distance parameter, revealed that local in-lake conditions are more strongly related to local macrophyte diversity than the distance to a propagule source (Rorslett, 1991; Vermonden et al., 2010). However, experimental or mechanistic tests of the role of the species pool and propagule pressure in determining macrophyte diversity are still lacking.
Furthermore, the role of food web interactions as determinants of macrophyte diversity remains largely unknown. Whereas connectivity improves propagule availability, which could improve macrophyte diversity, isolation is known to increase macrophyte diversity in ponds. An explanation for the counter-intuitive relationship between isolation and species richness is that isolated ponds frequently lack benthivorous fish, which create turbid conditions through their foraging in sediment. Isolated ponds that lack those fish have higher richness of macrophyte species because they become turbid less easily if habitat conditions deteriorate (Scheffer et al., 2006). However, at high macrophyte productivity, moderate levels of disturbance may actually increase macrophyte diversity. Herbivores that graze on the dominant plant species and create moderate sediment disturbance create recruitment opportunities for subordinate plants, thereby improving macrophyte diversity (Sandsten & Klaassen, 2008; Hidding et al., 2010b). Similarly, water level fluctuations can enhance macrophyte diversity (Rorslett, 1991): drawdowns particularly have been shown to improve richness of submerged macrophytes (Van Geest et al., 2005).
Restoration measures
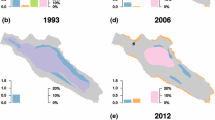
How do restoration measures affect the return of species rich vegetation? There are several well-documented examples of restoration projects where external nutrient loading has been reduced and the effects on the vegetation have been placed in a long-term context (Table 1). These examples show that reducing external nutrient loading does result in a return of macrophytes and an increase in species richness 20–40 years after peak nutrient loading, when macrophytes had almost disappeared (Table 1). Macrophyte return is slow and there can be a delay in recovery, where macrophytes do not yet colonise even though transparency has increased in response to reduced nutrient loading, a phenomenon also observed in other studies of nutrient reduction (Jeppesen et al., 2005a, b; Phillips et al., 2005). Furthermore, whereas macrophytes returned and species richness improved after reduction of nutrient loading, a longer term comparison shows that the species richness and macrophyte community is different from the records about a century ago, from the early 1900s, which was the start of human-induced large-scale eutrophication (Table 1). The authors suggest that this may be due to an impoverished regional species pool, where species are nowadays rare, altered sediment characteristics and competition from tall growing eutrophic species, which inhibits the return of smaller, rare, oligotrophic species (Sand-Jensen et al., 2008; Hilt et al., 2010; Dudley et al., 2012). This observation raises the question whether the changes to the aquatic habitat, particularly the sediment, and plant communities induced by eutrophication are reversible.
In contrast to the reduction of nutrient loading, biomanipulation can create an almost immediate response of both enhanced light availability and macrophyte growth (Lauridsen et al., 2003a, b; Table 1).
Clear water is the foremost requirement to allow optimal under-water light conditions for macrophytes to germinate and grow. In lakes where sediment contributes most to water turbidity and sediment dynamics prevent macrophyte recruitment, the creation of shelter and removal of sediment disturbing fish will be necessary; as shown by lake biomanipulation studies (Gulati & Van Donk, 2002; Sondergaard et al., 2007; Gulati et al., 2008). When optimal light conditions are re-established, plants can respond fast by germination and colonisation of the shallow water body (Van de Haterd & Ter Heerdt, 2007). However, whereas biomanipulation can initially result in a strong expansion of macrophyte vegetation, which can be species rich, the community often rapidly changes and becomes dominated by eutrophic species. On one hand the abundance of eutrophic species may enhance water transparency, but on the other hand the species that dominate and grow to the water surface will limit light deeper in the water column, which will reduce the number of species that can grow under these conditions (Sand-Jensen et al., 2008). Additionally, dominance by eutrophic species does often not result in a stable vegetation and a relapse to the turbid state can occur within 10 years (Sondergaard et al., 2008). To prevent the deterioration of the vegetation and clear water state, low nutrient levels are required, as indicated by the thresholds mentioned in the previous paragraph. To achieve such low nutrient levels, both external and internal nutrient loading should be reduced, depending on the nutrient loading of the sediment, for which many methods are available (Cooke et al., 1993; Hickey & Gibbs, 2009). Most of these measures are not harmful for the macrophyte habitat, apart from removal of nutrient-rich sediment. Even though decreased nutrient availability after dredging can result in clear water, it will also remove a large part of the macrophyte propagule bank. But, we still do not know if this hampers macrophyte recovery as the role of the propagule bank in restoring macrophyte vegetation remains largely unknown.
Water fluctuations will generally benefit the submerged vegetation by providing recruitment sites (Coops & Hosper, 2002). However, this also depends on the effects on water quality, as in some cases shallow water may be prone to algal blooms facilitated by the increased temperatures in a shallow water layer.
If there is uncertainty whether macrophyte species will colonise the restored areas and sustain the restored clear water state, planting them might be an option (Hilt et al., 2006). However, first the abiotic conditions for the growth of submerged macrophytes should be met as well as a reduction in the population of sediment disturbing fish or crustaceans including (invasive or stocked) crabs and crayfish. Subsequently one should wonder why macrophytes are not spontaneously returning to the restored water body. This may indicate that growing conditions are still not good enough and in that case transplanting will be unsuccessful. For macrophytes to maintain a clear water state a minimum coverage of the lake seems to be required; as a rule of thumb 30% coverage has been used as a minimum threshold (Jeppesen et al., 1994; Van Nes et al., 2002; Janse et al., 2008; Kosten et al., 2009a), which is in the range of 10%-40% reported by Sondergaard et al. (2010), but others report the need for higher coverage (50% Tatrai et al., 2009, 60% Blindow et al., 2002). In warm lakes in tropical and subtropical regions, a higher coverage of macrophytes may be needed as the grazing of zooplankton on phytoplankton is low due to high fish predation (Jeppesen et al., 2007; Kosten et al., 2009a). As this requires a tremendous effort, large-scale planting of macrophytes has not often been used. In China, subtropical Lake Huizhou (West Lake) has been planted completely with submerged macrophytes after removal of fish and has been clear for 6 years since planting it, with continued fish removal (Chen et al., 2010), even though it is assumed that (sub)tropical lakes are much harder to maintain in the clear water state (Jeppesen et al., 2005a, b).
Perspectives and conclusions
In view of the money spent and efforts put in restoration of submerged macrophytes, it is somewhat surprising that we still do not know exactly why the restoration of vegetation with high biodiversity fails in many lake restoration studies. In lake restoration, most attention has been paid to switch eutrophic lakes with highly turbid waters to a clear water state, with the assumption that the macrophyte vegetation will appear as soon as the water is clear and maintain this clear water state. However, the first bottleneck for lake restoration may be the absence of species either in the propagule bank, or in the form of relic populations that survived the period of unfavourable conditions. Currently, the intriguing question is: where do macrophytes come from after restoration? As long as we do not know how important propagule availability and dispersal are for the re-establishment of diverse macrophyte vegetation, it is not possible to take directed measures to improve the recruitment of a diverse vegetation other than creating the right abiotic conditions as is currently been attempted by many. We conclude that it is imperative to study the recruitment phase of macrophytes more closely for restoring diverse macrophyte communities as well as the biotic interactions including herbivory and plant competition. These essential study objectives will further determine the probability of macrophyte restoration and define what exactly can be restored and what not. The composition of the vegetation, in turn, affects the ecosystem functions that macrophytes have. A better understanding of the species specificity and of the importance of diversity of macrophyte vegetation in the fulfilling of ecosystem functions will both advance our knowledge of the role of macrophytes in shallow water bodies and lead to a better guidance of restoration efforts.
References
Al-Mufti, M. M., C. L. Sydes, S. B. Furness, J. P. Grime & S. R. Band, 1977. A quantitative analysis of shoot phenology and dominance in herbaceous vegetation. Journal of Ecology 65: 759–791.
Bakker, E. S., E. Van Donk, S. A. J. Declerck, N. R. Helmsing, B. Hidding & B. A. Nolet, 2010. Effect of macrophyte community composition and nutrient enrichment on plant biomass and algal blooms. Basic and Applied Ecology 11: 432–439.
Barker, T., K. Hatton, M. O’Connor, L. Connor & B. Moss, 2008. Effects of nitrate load on submerged plant biomass and species richness: results of a mesocosm experiment. Fundamental and Applied Limnology 173: 89–100.
Best, E. P. H., D. de Vries & A. Reins, 1984. Macrophytes in the Loosdrecht Lakes: a story of their decline in the course of eutrophication. Verhandlung Internationale Vereinigung Limnologie 22: 868–875.
Blindow, I., 1992a. Decline of charophytes during eutrophication: comparison with angiosperms. Freshwater Biology 28: 9–14.
Blindow, I., 1992b. Long- and short-term dynamics of submerged macrophytes in two shallow eutrophic lakes. Freshwater Biology 28: 15–27.
Blindow, I., A. Hargeby & G. Andersson, 2002. Seasonal changes of mechanisms maintaining clear water in a shallow lake with abundant Chara vegetation. Aquatic Botany 72: 315–334.
Boedeltje, G., G. N. J. ter Heerdt & J. P. Bakker, 2002. Applying the seedling-emergence method under waterlogged conditions to detect the seed bank of aquatic plants in submerged sediments. Aquatic Botany 72: 121–128.
Boedeltje, G., J. P. Bakker & G. N. J. ter Heerdt, 2003. Potential role of propagule banks in the development of aquatic vegetation in backwaters along navigation canals. Aquatic Botany 77: 53–69.
Bonis, A., J. Lepart & P. Grillas, 1995. Seed bank dynamics and coexistence of annual macrophytes in a temporary and variable habitat. Oikos 74: 81–92.
Bornette, G. & S. Puijalon, 2011. Response of aquatic plants to abiotic factors: a review 2011. Aquatic Sciences 73: 1–14.
Brochet, A. L., M. Guillemain, M. Gauthier-Clerc, H. Fritz & A. J. Green, 2010. Endozoochory of Mediterranean aquatic plant seeds by teal after a period of desiccation: Determinants of seed survival and influence of retention time on germinability and viability. Aquatic Botany 93: 99–106.
Brouwer, E. & J. G. M. Roelofs, 2001. Degraded softwater lakes: possibilities for restoration. Restoration Ecology 9: 155–166.
Brouwer, E., R. Bobbink & J. G. M. Roelofs, 2002. Restoration of aquatic macrophyte vegetation in acidified and eutrophied softwater lakes: an overview. Aquatic Botany 73: 405–431.
Burks, R. L., G. Mulderij, E. Gross, I. Jones, L. Jacobsen, E. Jeppesen & E. Van Donk, 2006. Center stage: the crucial role of macrophytes in regulating trophic interactions in shallow lake wetlands. In Bobbink, R., B. Beltman, J. T. A. Verhoeven & D. F. Whigham (eds), Wetlands: Functioning, Biodiversity Conservation, and Restoration. Springer, Berlin. Ecological Studies 191: 537–559.
Capers, R. S., 2003. Macrophyte colonization in a freshwater tidal wetland (Lyme, CT, USA). Aquatic Botany 77: 325–338.
Carpenter, S. R. & D. M. Lodge, 1986. Effects of submersed macrophytes on ecosystem processes. Aquatic Botany 26: 341–370.
Carpenter, S. R., N. F. Caraco, D. L. Correll, R. W. Howarth, A. N. Sharpley & V. H. Smith, 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8: 559–568.
Carvalho, L., C. Miller, B. M. Spears, I. D. M. Gunn, H. Bennion, A. Kirika & L. May, 2012. Water quality of Loch Leven: responses to enrichment, restoration and climate change. Hydrobiologia 681: 35–47.
Casanova, M. T. & M. A. Brock, 1990. Charophyte germination and establishment from the seed bank of an Australian temporatry lake. Aquatic Botany 36: 247–254.
Charalambidou, I. & L. Santamaria, 2005. Field evidence for the potential of waterbirds as dispersers of aquatic organisms. Wetlands 25: 252–258.
Chen, L., X. Zhang & Z. Liu, 2010. The response of the phytoplankton community to ecosystem restoration in Huizhou West Lake. Journal of Wuhan Botanical Research 28(4): 453–459. (in Chinese with an English abstract).
Cooke, G. D., E. B. Welch, S. A. Peterson & P. R. Newroth, 1993. Restoration and Management of Lakes and Reservoirs, 2nd ed. Lewis Publishers, Ann Arbor: 548 pp.
Coops, H. & S. H. Hosper, 2002. Water-level management as a tool for the restoration of shallow lakes in the Netherlands. Lake and Reservoir Management 18: 293–298.
De Winton, M. D., J. S. Clayton & P. D. Champion, 2000. Seedling emergence from seed banks of 15 New Zealand lakes with contrasting vegetation histories. Aquatic Botany 66: 181–194.
Declerck, S., J. Vandekerkhove, L. Johansson, K. Muylaert, J. M. Conde-Porcuna, K. Van der Gucht, C. Perez-Martinez, T. Lauridsen, K. Schwenk, G. Zwart, W. Rommens, J. Lopez-Ramos, E. Jeppesen, W. Vyverman, L. Brendonck & L. De Meester, 2005. Multi-group biodiversity in shallow lakes along gradients of phosphorus and water plant cover. Ecology 86: 1905–1915.
Declerck, S. A. J., E. S. Bakker, B. Van Lith, A. Kersbergen & E. Van Donk, 2011. Effects of nutrient additions and macrophyte composition on invertebrate community assembly and diversity in experimental ponds. Basic and Applied Ecology 12: 466–475.
Dorenbosch, M. & E. S. Bakker, 2011. Herbivory in omnivorous fishes: effect of presence of plant secondary metabolites and prey stoichiometry. Freshwater Biology 56: 1783–1797.
Dudley, B., I. D. M. Gunn, L. Carvalho, I. Proctor, M. T. O’Hare, K. J. Murphy & A. Milligan, 2012. Changes in aquatic macrophyte communities in Loch Leven: evidence of recovery from eutrophication? Hydrobiologia 681: 49–57.
Edvardsen, A. & R. H. Okland, 2006. Variation in plant species richness in and adjacent to 64 ponds in SE Norwegian agricultural landscapes. Aquatic Botany 85: 79–91.
Engelhardt, K. A. M. & M. E. Ritchie, 2001. Effects of macrophyte species richness on wetland ecosystem functioning and services. Nature 411: 687–689.
Espinar, J. L. & L. Clemente, 2007. The impact of vertic soil cracks on submerged macrophyte diaspora bank depth distribution in Mediterranean temporary wetlands. Aquatic Botany 87: 325–328.
Feuchtmayr, H., R. Moran, K. Hatton, L. Connor, T. Heyes, B. Moss, I. Harvey & D. Atkinson, 2009. Global warming and eutrophication: effects on water chemistry and autotrophic communities in experimental hypertrophic shallow lake mesocosms. Journal of Applied Ecology 46: 713–723.
Figuerola, J., I. Charalambidou, L. Santamaria & A. J. Green, 2010. Internal dispersal of seeds by waterfowl: effect of seed size on gut passage time and germination patterns. Naturwissenschaften 97: 555–565.
Geurts, J. J. M., 2010. Restoration of fens and peat lakes: a biogeochemical approach. PhD-thesis, Radboud University, Nijmegen: 170 pp.
Geurts, J. J. M., A. J. P. Smolders, J. T. A. Verhoeven, J. G. M. Roelofs & L. P. M. Lamers, 2008. Sediment Fe:PO4 ratio as a diagnostic and prognostic tool for the restoration of macrophyte biodiversity in fen waters. Freshwater Biology 53: 2101–2116.
Green, A. J., J. Figuerola & M. I. Sanchez, 2002. Implications of waterbird ecology for the dispersal of aquatic organisms. Acta Oecologia-International Journal of Ecology 23: 177–189.
Grillas, P., P. Garcia-Murillo, O. Geertz-Hansen, N. Marbá, C. Montes, C. M. Duarte, L. Tan Ham & A. Grossmann, 1993. Submerged macrophyte seed bank in a Mediterranean temporary marsh: abundance and relationship with established vegetation. Oecologia 94: 1–6.
Gulati, R. D. & E. van Donk, 2002. Lakes in the Netherlands, their origin, eutrophication and restoration: state-of-the-art review. Hydrobiologia 478: 73–106.
Gulati, R. D., L. M. D. Pires & E. van Donk, 2008. Lake restoration studies: failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 38: 233–247.
Guo, L., 2007. Ecology. Doing battle with the green monster of Taihu Lake. Science 317: 1166.
Hargeby, A., I. Blindow & G. Andersson, 2007. Long-term patterns of shifts between clear and turbid states in Lake Krankesjon and Lake Takern. Ecosystems 10: 28–35.
Harwell, M. C. & K. E. Havens, 2003. Experimental studies on the recovery potential of submerged aquatic vegetation after flooding and desiccation in a large subtropical lake. Aquatic Botany 77: 135–151.
Hickey, C. W. & M. M. Gibbs, 2009. Lake sediment phosphorus release management—decision support and risk assessment framework. New Zealand Journal of Marine and Freshwater Research 43: 819–856.
Hidding, B., E. S. Bakker, F. Keuper, T. de Boer, P. P. de Vries & B. A. Nolet, 2010a. Differences in tolerance of pondweeds and charophytes to vertebrate herbivores in a shallow Baltic estuary. Aquatic Botany 93: 123–128.
Hidding, B., B. A. Nolet, T. de Boer, P. P. de Vries & M. Klaassen, 2010b. Above- and below-ground vertebrate herbivory may each favour a different subordinate species in an aquatic plant community. Oecologia 162: 199–208.
Hilt, S., 2006. Recovery of Potamogeton pectinatus L. stands in a shallow eutrophic lake under extreme grazing pressure. Hydrobiologia 570: 95–99.
Hilt, S. & E. M. Gross, 2008. Can allelopathically active submerged macrophytes stabilise clear-water states in shallow lakes? Basic and Applied Ecology 9: 422–432.
Hilt, S., E. M. Gross, M. Hupfer, H. Morscheid, J. Mahlmann, A. Melzer, J. Poltz, S. Sandrock, E. M. Scharf, S. Schneider & K. van de Weyer, 2006. Restoration of submerged vegetation in shallow eutrophic lakes—a guideline and state of the art in Germany. Limnologica 36: 155–171.
Hilt, S., K. Van de Weyer, A. Kohler & I. Chorus, 2010. Submerged macrophyte responses to reduced phosphorus concentrations in two peri-urban lakes. Restoration Ecology 18: 452–461.
Ibelings, B. W., R. Portielje, E. H. R. R. Lammens, R. Noordhuis, M. S. Van den Berg, W. Joosse & M. L. Meijer, 2007. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems 10: 4–16.
Irfanullah, H. M. & B. Moss, 2004. Factors influencing the return of submerged plants to a clear-water, shallow temperate lake. Aquatic Botany 80: 177–191.
James, C., J. Fisher, V. Russell, S. Collings & B. Moss, 2005. Nitrate availability and hydrophyte species richness in shallow lakes. Freshwater Biology 50: 1049–1063.
Janse, J. H., L. N. De Senerpont Domis, M. Scheffer, L. Lijklema, L. Van Liere, M. Klinge & W. M. Mooij, 2008. Critical phosphorus loading of different types of shallow lakes and the consequences for management estimated with the ecosystem model PCLake. Limnologica 38: 203–219.
Jeppesen, E., M. Sondergaard, E. Kanstrup, B. Petersen, R. B. Eriksen, M. Hammershøj, E. Mortensen, J. P. Jensen & A. Have, 1994. Does the impact of nutrients on the biological structure and function of brackish and freshwater lakes differ? Hydrobiologia 275(276): 15–30.
Jeppesen, E. J. P. Jensen, M. Sondergaard, T. L. Lauridsen, L. J. Pedersen & L. Jensen, 1997. Top-down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342(343): 151–164.
Jeppesen, E., M. Sondergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. Foy, D. Gerdeaux, S. E. Hampton, S. Hilt, K. Kangur, J. Köhler, E. H. H. R. Lammes, T. L. Lauridsen, M. Manca, M. R. Miracle, B. Moss, P. Nõges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willen & M. Winder, 2005a. Lake responses to reduced nutrient loading—an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771.
Jeppesen, E., M. Sondergaard, N. Mazzeo, M. Meerhoff, C. Branco, V. Huszar & F. Scasso, 2005b. Lake restoration and biomanipulation in temperate lakes: relevance for subtropical and tropical lakes. In Reddy, M. V. (ed.), Restoration and Management of Tropical Eutrophic Lakes. Oxford & IBH Publishing Co. Pvt. Ltd., New Delhi: 331–349.
Jeppesen, E., M. Meerhoff, B. A. Jacobsen, R. S. Hansen, M. Sondergaard, J. P. Jensen, T. L. Lauridsen, N. Mazzeo & C. W. C. Branco, 2007. Restoration of shallow lakes by nutrient control and biomanipulation—the successful strategy varies with lake size and climate. Hydrobiologia 581: 269–285.
Jones, J. I. & C. D. Sayer, 2003. Does the fish-invertebrate-periphyton cascade precipitate plant loss in shallow lakes? Ecology 84: 2155–2167.
Kleyer, M., R. M. Bekker, I. C. Knevel, J. P. Bakker, K. Thompson, M. Sonnenschein, P. Poschlod, J. M. Van Groenendael, L. Klimes, J. Klimesová, S. Klotz, G. M. Rusch, M. Hermy, D. Adriaens, G. Boedeltje, B. Bossuyt, A. Dannemann, P. Endels, L. Götzenberger, J. G. Hodgson, A.-K. Jackel, I. Kühn, D. Kunzmann, W. A. Ozinga, C. Römermann, M. Stadler, J. Schlegelmilch, H. J. Steendam, O. Tackenberg, B. Wilmann, H. J. C. Cornelissen, O. Eriksson, E. Garnier & B. Peco, 2008. The LEDA Traitbase: a database of life-history traits of Northwest European flora. Journal of Ecology 96: 1266–1274.
Korner, S., 2001. Development of submerged macrophytes in shallow Lake Muggelsee (Berlin, Germany) before and after its switch to the phytoplankton-dominated state. Archives of Hydrobiology 152: 395–409.
Kosten, S., A. Kamarainen, A. Jeppesen, E. H. Van Nes, E. T. H. M. Peeters, N. Mazzeo, L. Sass, J. Hauxwell, N. Hansel-Welch, T. L. Lauridsen, M. Sondergaard, R. W. Bachmann, G. Lacerot & M. Scheffer, 2009a. Climate-related differences in the dominance of submerged macrophytes in shallow lakes. Global Change Biology 15: 2503–2517.
Kosten, S., G. Lacerot, E. Jeppesen, D. D. Marques, E. H. Van Nes, N. Mazzeo & M. Scheffer, 2009b. Effects of submerged vegetation on water clarity across climates. Ecosystems 12: 1117–1129.
Kufel, L. & I. Kufel, 2002. Chara beds acting as nutrient sinks in shallow lakes—a review. Aquatic Botany 72: 249–260.
Lambert, S. J. & A. J. Davy, 2011. Water quality as a threat to aquatic plants: discriminating between the effects of nitrate, phosphate, boron and heavy metals on charophytes. New Phytologist 189: 1051–1059.
Lamers, L. P. M., A. J. P. Smolders & J. G. M. Roelofs, 2002. The restoration of fens in the Netherlands. Hydrobiologia 478: 107–130.
Lauridsen, T., E. Jeppesen & F. O. Andersen, 1993. Colonization of submerged macrophytes in shallow fish manipulated Lake Vaeng—impact of sediment composition and waterfowl grazing. Aquatic Botany 46: 1–15.
Lauridsen, T. L., J. P. Jensen, E. Jeppesen & M. Søndergaard, 2003a. Response of submerged macrophytes in Danish lakes to nutrient loading reductions and biomanipulation. Hydrobiologia 506–509: 641–649.
Lauridsen, T. L., H. Sandsten & P. Hald Møller, 2003b. Restoration of a shallow lake by introducing Potamogeton spp.: impact of waterfowl grazing. Lakes & Reservoirs: Research and Management 8: 177–187.
Li, E. H., G. H. Liu, W. Li, L. Y. Yuan & S. C. Li, 2008. The seed-bank of a lakeshore wetland in Lake Honghu: implications for restoration. Plant Ecology 195: 69–76.
Liu, G. H., W. Li, J. Zhou, W. Z. Liu, D. Yang & A. J. Davy, 2006. How does the propagule bank contribute to cyclic vegetation change in a lakeshore marsh with seasonal drawdown? Aquatic Botany 84: 137–143.
Liu, W., Q. Zhang & G. Liu, 2009. Seed banks of a river–reservoir wetland system and their implications for vegetation development. Aquatic Botany 90: 7–12.
Lombardo, P. & G. D. Cooke, 2003. Ceratophyllum demersum–phosphorus interactions in nutrient enriched aquaria. Hydrobiologia 497: 79–90.
Makela, S., E. Huitu & L. Arvola, 2004. Spatial patterns in aquatic vegetation composition and environmental covariates along chains of lakes in the Kokemdenjoki watershed (S. Finland). Aquatic Botany 80: 253–269.
Marklund, O., H. Sandsten, L. A. Hansson & I. Blindow, 2002. Effects of waterfowl and fish on submerged vegetation and macroinvertebrates. Freshwater Biology 47: 2049–2059.
McAbendroth, L., P. M. Ramsay, A. Foggo, S. D. Rundle & D. T. Bilton, 2005. Does macrophyte fractal complexity drive invertebrate diversity, biomass and body size distributions? Oikos 111: 279–290.
Moore, K. A., E. C. Shields & J. C. Jarvis, 2010. The role of habitat and herbivory on the restoration of tidal freshwater submerged aquatic vegetation populations. Restoration Ecology 18: 596–604.
Moss, B., 1989. Water pollution and the management of ecosystems: a case study of science and scientist. In Grubb, P. J. & J. B. Whittaker (eds), Towards a More Exact Ecology. Blackwell, Oxford: 401–422.
Mulderij, G., E. van Donk & J. G. M. Roelofs, 2003. Differential sensitivity of green algae to allelopathic substances from Chara. Hydrobiologia 491: 261–271.
Murphy, K. J., 2002. Plant communities and plant diversity in softwater lakes of northern Europe. Aquatic Botany 73: 287–324.
Nagasaka, M., 2004. Changes in biomass and spatial distribution of Elodea nuttallii (Planch.) St. John, an invasive submerged plant, in oligomesotrophic Lake Kizaki from 1999 to 2002. Limnology 5: 129–139.
Nilsson, C., R. L. Brown, R. Jansson & D. M. Merritt, 2010. The role of hydrochory in structuring riparian and wetland vegetation. Biological Reviews 85: 837–858.
Noordhuis, R., D. T. Van der Molen & M. S. Van den Berg, 2002. Response of herbivorous water-birds to the return of Chara in Lake Veluwemeer, the Netherlands. Aquatic Botany 72: 349–367.
Ozimek, T., 2006. The possibility of submerged macrophyte recovery from a propagule bank in the eutrophic Lake Mikołajskie (North Poland). Hydrobiologia 570: 127–131.
Penning, W. E., M. Mjelde, B. Dudley, S. Hellsten, J. Hanganu, A. Kolada, M. Van den Berg, S. Poikane, G. Phillips, N. Willby & F. Ecke, 2008. Classifying aquatic macrophytes as indicators of eutrophication in European lakes. Aquatic Ecology 42: 237–251.
Perrow, M. R., J. H. Schutten, J. R. Howes, T. Holzer, F. J. Madgwick & A. J. D. Jowitt, 1997. Interactions between coot (Fulica atra) and submerged macrophytes: the role of birds in the restoration process. Hydrobiologia 342: 241–255.
Phillips, G. L., D. Eminson & B. Moss, 1978. A mechanism to account for macrophyte decline in progressively eutrophicated freshwaters. Aquatic Botany 4: 103–126.
Phillips, G., A. Kelly, J.-A. Pitt, R. Sanderson & E. Taylor, 2005. The recovery of a very shallow eutrophic lake, 20 years after the control of effluent derived phosphorus. Freshwater Biology 50: 1628–1638.
Pollux, B. J. A., 2011. The experimental study of seed dispersal by fish (ichthyochory). Freshwater Biology 56: 197–212.
Pollux, B. J. A., A. Luteijn, J. M. Van Groenendael & N. J. Ouborg, 2009. Gene flow and genetic structure of the aquatic macrophyte Sparganium emersum in a linear unidirectional river. Freshwater Biology 54: 64–76.
Porter, J. L., R. T. Kingsford & M. A. Brock, 2007. Seed banks in arid wetlands with contrasting flooding, salinity and turbidity regimes. Plant Ecology 188: 215–234.
Portielje, R. & R. M. M. Roijackers, 1995. Primary succession of aquatic macrophytes in experimental ditches in relation to nutrient input. Aquatic Botany 50: 127–140.
Riddin, T. & J. B. Adams, 2009. The seed banks of two temporarily open/closed estuaries in South Africa. Aquatic Botany 90: 328–332.
Roberts, E., J. Kroker, S. Korner & A. Nicklisch, 2003. The role of periphyton during the re-colonization of a shallow lake with submerged macrophytes. Hydrobiologia 506: 525–530.
Rorslett, B., 1991. Principal determinants of aquatic macrophyte richness in northern European lakes. Aquatic Botany 39: 173–193.
Sand-Jensen, K., T. Riis, O. Vestergaard & S. E. Larsen, 2000. Macrophyte decline in Danish lakes and streams over the past 100 years. Journal of Ecology 88: 1030–1040.
Sand-Jensen, K., N. L. Pedersen, I. Thorsgaard, B. Moeslund, J. Borum & K. P. Brodersen, 2008. 100 years of vegetation decline and recovery in Lake Fure, Denmark. Journal of Ecology 96: 206–271.
Sandsten, H. & M. Klaassen, 2008. Swan foraging shapes spatial distribution of two submerged plants, favouring the preferred prey species. Oecologia 156: 569–576.
Santamaria, L. & A. I. L. Garcia, 2004. Latitudinal variation in tuber production in an aquatic pseudo-annual plant, Potamogeton pectinatus. Aquatic Botany 79: 51–64.
Sarneel, J. M., 2010. Colonisation processes in riparian fen vegetation. PhD thesis, Utrecht University, Utrecht.
Sarneel, J. M., M. B. Soons, J. J. M. Geurts, B. Beltman & J. T. A. Verhoeven, 2011. Multiple effects of land-use changes impede the colonization of open water in fen ponds. Journal of Vegetation Science 22: 551–563.
Sayer, S. D., T. A. Davidson & J. I. Jones, 2010. Seasonal dynamics of macrophytes and phytoplankton in shallow lakes: a eutrophication-driven pathway from plants to plankton? Freshwater Biology 55: 500–513.
Scheffer, M., S. H. Hosper, M.-L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Scheffer, M., M. Van den Berg, A. Breukelaar, C. Breukers, H. Coops, R. Doef & M. L. Meijer, 1994. Vegetated areas with clear water in turbid shallow lakes. Aquatic Botany 49: 193–196.
Scheffer, M., S. Carpenter, J. A. Foley, C. Folke & B. Walker, 2001. Catastrophic shifts in ecosystems. Nature 413: 591–596.
Scheffer, M., G. J. Van Geest, K. Zimmer, E. Jeppesen, M. Sondergaard, M. G. Butler, M. A. Hanson, S. De Clerck & L. De Meester, 2006. Small habitat size and isolation can promote species richness: second-order effects on biodiversity in shallow lakes and ponds. Oikos 112: 227–231.
Sederias, J. & B. Colman, 2007. The interaction of light and low temperature on breaking the dormancy of Chara vulgaris oospores. Aquatic Botany 87: 229–234.
Sederias, J. & B. Colman, 2009. Inhibition of Chara vulgaris oospore germination by sulfidic sediments. Aquatic Botany 91: 273–278.
Smolders, A. J. P. & J. G. M. Roelofs, 1996. The roles of internal iron hydroxide precipitation, sulphide toxicity and oxidizing ability in the survival of Stratiotes aloides roots at different iron concentrations in sediment pore water. New Phytologist 133: 253–260.
Sondergaard, M., L. Bruun, T. Lauridsen, E. Jeppesen & T. V. Madsen, 1996. The impact of grazing waterfowl on submerged macrophytes: in situ experiments in a shallow eutrophic lake. Aquatic Botany 53: 73–84.
Sondergaard, M., E. Jeppesen, T. L. Lauridsen, C. Skov, E. H. Van Nes, R. Roijackers, E. Lammens & R. Portielje, 2007. Lake restoration: successes, failures and long-term effects. Journal of Applied Ecology 44: 1095–1105.
Sondergaard, M., L. Liboriussen, A. R. Pedersen & E. Jeppesen, 2008. Lake restoration by fish removal: short- and long-term effects in 36 Danish lakes. Ecosystems 11: 1291–1305.
Sondergaard, M., L. S. Johansson, T. L. Lauridsen, T. B. Jorgensen, L. Liboriussen & E. Jeppesen, 2010. Submerged macrophytes as indicators of the ecological quality of lakes. Freshwater Biology 55: 893–908.
Soomers, H., D. N. Winkel, Y. Du & M. J. Wassen, 2010. The dispersal and deposition of hydrochorous plant seeds in drainage ditches. Freshwater Biology 55: 2032–2046.
Soons, M. B., C. van der Vlugt, B. van Lith, G. W. Heil & M. Klaassen, 2008. Small seed size increases the potential for dispersal of wetland plants by ducks. Journal of Ecology 96: 619–627.
Spencer, D. F. & G. G. Ksander, 2002. Sedimentation disrupts natural regeneration of Zannichellia palustris in Fall River, California. Aquatic Botany 73: 137–147.
Stiers, I., N. Crohain, G. Josens & L. Triest, 2011. Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds. Biological Invasions 13: 2715–2726.
Strand, J. A. & S. E. B. Weisner, 2001. Dynamics of submerged macrophyte populations in response to biomanipulation. Freshwater Biology 46: 1397–1408.
Tatrai, I., G. Boros, A. Gyorgy, K. Matyas, J. Korponai, P. Pomogyi, M. Havasi & T. Kucserka, 2009. Abrupt shift from clear to turbid state in a shallow eutrophic, biomanipulated lake. Hydrobiologia 620: 149–161.
Ter Heerdt, G. & M. Hootsmans, 2007. Why biomanipulation can be effective in peaty lakes. Hydrobiologia 584: 305–316.
Tilman, D., J. Fargione, B. Wolff, C. D’Antonio, A. Dobson, R. Howarth, D. Schindler, W. H. Schlesinger, D. Simberloff & D. Swackhamer, 2001. Forecasting agriculturally driven global environmental change. Science 292: 281–284.
Van de Bund, W. J. & E. van Donk, 2002. Short-term and long-term effects of zooplanktivorous fish removal in a shallow lake: a synthesis of 15 years of data from Lake Zwemlust. Freshwater Biology 47: 2380–2387.
Van de Haterd, R. J. W. & G. N. J. Ter Heerdt, 2007. Potential for the development of submerged macrophytes in eutrophicated shallow peaty lakes after restoration measures. Hydrobiologia 584: 277–290.
Van den Berg, M. S., M. Scheffer, E. Van Nes & H. Coops, 1999. Dynamics and stability of Chara sp. and Potamogeton pectinatus in a shallow lake changing in eutrophication level. Hydrobiologia 408: 335–342.
Van den Berg, M. S., H. Coops & J. Simons, 2001. Propagule bank buildup of Chara aspera and its significance for colonization of a shallow lake. Hydrobiologia 462: 9–17.
Van der Welle, M. E. W., M. Cuppens, L. P. M. Lamers & J. G. M. Roelofs, 2006. Detoxifying toxicants: interactions between sulfide and iron toxicity in freshwater wetlands. Environmental Toxicology and Chemistry 25: 1592–1597.
Van der Welle, M. E. W., A. J. P. Smolders, H. J. M. O. Den Camp, J. G. M. Roelofs & L. P. M. Lamers, 2007. Biogeochemical interactions between iron and sulphate in freshwater wetlands and their implications for interspecific competition between aquatic macrophytes. Freshwater Biology 52: 434–447.
Van Donk, E. & A. Otte, 1996. Effects of grazing by fish and waterfowl on the biomass and species composition of submerged macrophytes. Hydrobiologia 340: 285–290.
Van Donk, E. & W. J. Van de Bund, 2002. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms. Aquatic Botany 72: 261–274.
Van Geest, G. J., H. Wolters, F. C. J. M. Roozen, H. Coops, R. M. M. Roijackers, A. D. Buijse & M. Scheffer, 2005. Water-level fluctuations affect macrophyte richness in floodplain lakes. Hydrobiologia 539: 239–248.
Van Nes, E. H., M. Scheffer, M. S. Van den Berg & H. Coops, 2002. Dominance of charophytes in eutrophic shallow lakes—when should we expect it to be an alternative stable state? Aquatic Botany 72: 275–296.
Vermaat, J. E., L. Santamaria & P. J. Roos, 2000. Water flow across and sediment trapping in submerged macrophyte beds of contrasting growth form. Archiv für Hydrobiologie 148: 549–562.
Vermonden, K., R. S. E. W. Leuven, G. van der Velde, H. A. J. Hendriks, M. M. van Katwijk, J. G. M. Roelofs, E. C. H. E. T. Lucassen, O. Pedersen & K. Sand-Jensen, 2010. Species pool versus site limitations of macrophytes in urban waters. Aquatic Sciences 72: 379–389.
Vestergaard, O. & K. Sand-Jensen, 2000. Alkalinity and trophic state regulate aquatic plant distribution in Danish lakes. Aquatic Botany 67: 85–107.
Weisner, S. E. B., J. A. Strand & H. Sandsten, 1997. Mechanisms regulating abundance of submerged vegetation in shallow eutrophic lakes. Oecologia 109: 592–599.
Willby, N. J., J. R. Pygott & J. W. Eaton, 2001. Inter-relationships between standing crop, biodiversity and trait attributes of hydrophytic vegetation in artificial waterways. Freshwater Biology 46: 883–902.
Xiao, C., W. F. Dou & G. H. Liu, 2010. Variation in vegetation and seed banks of freshwater lakes with contrasting intensity of aquaculture along the Yangtze River, China. Aquatic Botany 92: 195–199.
Xie, D., D. Yu, L. F. Yu & C. H. Liu, 2010. Asexual propagations of introduced exotic macrophytes Elodea nuttallii, Myriophyllum aquaticum, and M. propinquum are improved by nutrient-rich sediments in China. Hydrobiologia 655: 37–47.
Acknowledgments
We thank two anonymous reviewers for their useful comments on a previous version of our paper. This is publication 5284 of the Netherlands Institute of Ecology (NIOO-KNAW).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Zhengwen Liu, Bo-Ping Han & Ramesh D. Gulati / Conservation, management and restoration of shallow lake ecosystems facing multiple stressors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bakker, E.S., Sarneel, J.M., Gulati, R.D. et al. Restoring macrophyte diversity in shallow temperate lakes: biotic versus abiotic constraints. Hydrobiologia 710, 23–37 (2013). https://doi.org/10.1007/s10750-012-1142-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1142-9