Summary
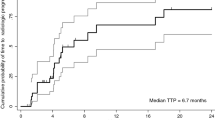
Background and Rationale Bortezomib (PS-341, VELCADE®) is a selective inhibitor of the 26S proteasome, an integral component of the ubiquitin-proteasome pathway. This phase II study evaluated the activity and tolerability of bortezomib in unresectable hepatocellular carcinoma (HCC) patients. Methods The primary endpoint was confirmed tumor response rate (RR) with secondary endpoints including duration of response, time to disease progression, survival and toxicity. Treatment consisted of bortezomib, 1.3 mg/m2 IV bolus on days 1, 4, 8, and 11 of each 21-day treatment cycle. Eligibility included: no prior systemic chemotherapy, ECOG PS 0-2, Child-Pugh A or B, preserved hematologic, hepatic and neurologic function; prior liver-directed therapy was permitted. Results Thirty-five patients enrolled and received a median of 2 cycles of treatment (range 1–12). Overall, 24 and 4 patients had a maximum severity of grade 3 and 4 adverse events (AEs), respectively. No treatment related deaths occurred. Only thrombocytopenia (11%) was seen in greater than 10% of patients. One patient achieved a partial response, lasting 13 weeks during treatment and progressed 11.6 months later; two patients received treatment for greater than 6 months. Median time-to-progression was 1.6 months and median survival was 6.0 months. Conclusions This international, multicenter trial evaluated bortezomib as monotherapy in unresectable HCC patients. And, despite the lack of significant activity, this report serves as a baseline clinical experience for the development of future dual biologic approaches including bortezomib.


Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- RR:
-
Response rate
- AEs:
-
Adverse events
- VEGF:
-
Vascular endothelial growth factor
- UNL:
-
Upper normal limit
- CTCAE:
-
Common terminology criteria for adverse events
- CBCs:
-
Complete blood cell counts
- CR:
-
Complete response
- PR:
-
Partial response
- PD:
-
Progressive disease
- SD:
-
Stable disease
- CIs:
-
Confidence intervals
- SHARP:
-
Sorafenib hepatocellular carcinoma assessment randomized protocol
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the united states. N Engl J Med 340(10):745–750. doi:10.1056/nejm199903113401001
El-Serag HB (2007) Epidemiology of hepatocellular carcinoma in USA. Hepatol Res 37(Suppl 2):S88–94
Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, Taylor I, Moscovici M, Saltz LB (2006) Phase ii study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24(26):4293–4300
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Cheng A, Kang Y, Chen Z, Tsao C, Qin S, Kim J, Burock K, Zou J, Voliotis D, Guan ZZ (2008) Randomized phase iii trial of sorafenib versus placebo in asian patients with advanced hepatocellular carcinoma. J Clin Oncol (Meeting Abstracts) 26 (15_suppl):4509
Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J (1999) The proteasome inhibitor ps-341 in cancer therapy. Clin Cancer Res 5(9):2638–2645
Rolfe M, Chiu MI, Pagano M (1997) The ubiquitin-mediated proteolytic pathway as a therapeutic area. J Mol Med 75(1):5–17
Ciechanover A, Orian A, Schwartz AL (2000) Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 22(5):442–451
Ozturk M (1999) Genetic aspects of hepatocellular carcinogenesis. Semin Liver Dis 19(3):235–242
Wang XW, Forrester K, Yeh H, Feitelson MA, Gu JR, Harris CC (1994) Hepatitis b virus x protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ercc3. Proc Natl Acad Sci USA 91(6):2230–2234
Ray RB, Steele R, Meyer K, Ray R (1997) Transcriptional repression of p53 promoter by hepatitis c virus core protein. J Biol Chem 272(17):10983–10986
Frankel A, Man S, Elliott P, Adams J, Kerbel RS (2000) Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor ps-341. Clin Cancer Res 6(9):3719–3728
Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, Adams J, Elliott P, Van Waes C (2001) Novel proteasome inhibitor ps-341 inhibits activation of nuclear factor-kappa b, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res 7(5):1419–1428
Cusack JC Jr, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, Baldwin AS Jr (2001) Enhanced chemosensitivity to cpt-11 with proteasome inhibitor ps-341: Implications for systemic nuclear factor-kappab inhibition. Cancer Res 61(9):3535–3540
Bold RJ, Virudachalam S, McConkey DJ (2001) Chemosensitization of pancreatic cancer by inhibition of the 26s proteasome. J Surg Res 100(1):11–17
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos M-V, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG, The VTI (2008) Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 359(9):906–917. doi:10.1056/NEJMoa0801479
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348(26):2609–2617
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst 92(3):205–216
Simon R (1989) Optimal two-stage designs for phase ii clinical trials. Control Clin Trials 10(1):1–10
Duffy D, Santner T (1987) Confidence intervals for a binomial parameter based on multistage tests. Biometrics 43:81–93
Kaplan MP (1958) Nonparametirc estimation for incomplete observations. J Am Stat Assoc 53:457–481
Chang L, Kamata H, Solinas G, Luo J-L, Maeda S, Venuprasad K, Liu Y-C, Karin M (2006) The e3 ubiquitin ligase itch couples jnk activation to tnf[alpha]-induced cell death by inducing c-flipl turnover. Cell 124(3):601–613
Anan A, Baskin-Bey ES, Isomoto H, Mott JL, Bronk SF, Albrecht JH, Gores GJ (2006) Proteasome inhibition attenuates hepatic injury in the bile duct-ligated mouse. Am J Physiol Gastrointest Liver Physiol 291(4):G709–716. doi:10.1152/ajpgi.00126.2006
Davis NB, Taber DA, Ansari RH, Ryan CW, George C, Vokes EE, Vogelzang NJ, Stadler WM (2004) Phase ii trial of ps-341 in patients with renal cell cancer: a university of chicago phase ii consortium study. J Clin Oncol 22(1):115–119. doi:10.1200/jco.2004.07.165
Engel RH, Brown JA, Von Roenn JH, O’Regan RM, Bergan R, Badve S, Rademaker A, Gradishar WJ (2007) A phase ii study of single agent bortezomib in patients with metastatic breast cancer: a single institution experience. Cancer Investig 25(8):733–737
Mackay H, Hedley D, Major P, Townsley C, Mackenzie M, Vincent M, Degendorfer P, Tsao M-S, Nicklee T, Birle D, Wright J, Siu L, Moore M, Oza A (2005) A phase ii trial with pharmacodynamic endpoints of the proteasome inhibitor bortezomib in patients with metastatic colorectal cancer. Clin Cancer Res 11(15):5526–5533. doi:10.1158/1078-0432.ccr-05-0081
Kozuch PS, Rocha-Lima CM, Dragovich T, Hochster H, O’Neil BH, Atiq OT, Pipas JM, Ryan DP, Lenz H-J (2008) Bortezomib with or without irinotecan in relapsed or refractory colorectal cancer: results from a randomized phase ii study. J Clin Oncol 26(14):2320–2326. doi:10.1200/jco.2007.14.0152
Alberts SR, Foster NR, Morton RF, Kugler J, Schaefer P, Wiesenfeld M, Fitch TR, Steen P, Kim GP, Gill S (2005) Ps-341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: a north central cancer treatment group (ncctg) randomized phase ii study. Ann Oncol 16(10):1654–1661
Hegewisch-Becker S, Sterneck M, Schubert U, Rogiers X, Guerciolini R, Pierce JE, Hossfeld DK (2004) Phase i/ii trial of bortezomib in patients with unresectable hepatocellular carcinoma (hcc). J Clin Oncol (Meeting Abstracts) 22 (14_suppl):4089
Berlin JD, Powell ME, Su Y, Horton L, Short S, Richmond A, Kauh JS, Staley CA, Mulcahy M, Benson AB, III (2008) Bortezomib (b) and doxorubicin (dox) in patients (pts) with hepatocellular cancer (hcc): A phase ii trial of the eastern cooperative oncology group (ecog 6202) with laboratory correlates. J Clin Oncol (Meeting Abstracts) 26 (15_suppl):4592
Acknowledgement
Supported by the Phase 2 Consortium Contract (NCI N01 CM17104).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, G.P., Mahoney, M.R., Szydlo, D. et al. An international, multicenter phase II trial of bortezomib in patients with hepatocellular carcinoma. Invest New Drugs 30, 387–394 (2012). https://doi.org/10.1007/s10637-010-9532-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9532-1