Abstract
Background
Experiments have reported that granulocyte colony stimulating factor (G-CSF) can mobilize stem cells. However, few studies have examined the effect of G-CSF on bone marrow mononuclear cell (BMMC) mobilization, in particular regarding their capability to home to acutely injured liver.
Aims
The aim of this study was to evaluate the effort of G-CSF on BMMC homing to the liver following chemically-induced hepatic failure.
Methods
BMMC were isolated from mice, pre-labeled with PKH26 and infused into the mice in which hepatic injury had been induced followed by administration of G-CSF or vehicle. Livers were studied by fluorescent microscopy after transplantation of pre-labeled BMMC.
Results
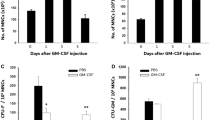
PKH26 labeled cells were found in liver tissue at 102 ± 10 cells/high power field in the BMMC+G-CSF group and 30 ± 5 cells/high power field in the BMMC group, but none in the G-CSF group and the control group (P < 0.05). In the former two groups the majority of PKH26 labeled cells colocalized with proliferative cell nuclear antigen (PCNA). The number of PCNA positive cells in the BMMC+G-CSF group was 20 ± 4 cells/high power field, while in the BMMC group it was 14 ± 2 cells/high power field, in the G-CSF group 12 ± 2 cells/high power field, and 8 ± 1 cells/high power field in the control group. Moreover, albumin expression was increased in the BMMC+G-CSF treated group (149 ± 7/high power field) relative to the BMMC group (48 ± 6/high power field), the G-CSF group (44 ± 5/high power field) and the vehicle group (30 ± 6/high power field), with the former three groups showing elevated levels as compared to vehicle control (30 ± 6) (P < 0.05).
Conclusion
Transplanted BMMC may home to injured liver, which appears to be enhanced by G-CSF administration.







Similar content being viewed by others
References
Masson S, Harrison DJ, Plevris JN, et al. Potential of hematopoietic stem cell therapy in hepatology: a critical review. Stem Cells. 2004;22:897–907.
Yannaki E, Athanasiou E, Xagorari A, et al. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108–119.
Thorgeirsson SS, Grisham JW. Overview of recent experimental studies on liver stem cells. Semin Liver Dis. 2003;23:303–312.
Tarella C, Zallio F, Caracciolo D, et al. Hemopoietic progenitor cell mobilization and harvest following an intensive chemotherapy debulking in indolent lymphoma patients. Stem Cells. 1999;17:55–61.
Di Campli C, Piscaglia AC, Giuliante F, et al. No evidence of hematopoietic stem cell mobilization in patients submitted to hepatectomy or in patients with acute on chronic liver failure. Transplant Proc. 2005;37:2563–2566.
Gaia S, Smedile A, Omede P, et al. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13–19.
Fukuhara S, Tomita S, Nakatani T, et al. G-CSF promotes bone marrow cells to migrate into infarcted mice heart, and differentiate into cardiomyocytes. Cell Transplant. 2004;13:741–748.
in ‘t Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–852.
Jin SZ, Meng XW, Han MZ, et al. Stromal cell derived factor-1 enhances bone marrow mononuclear cell migration in mice with acute liver failure. World J Gastroenterol. 2009;15:2657–2664.
Petersen BE, Zajac VF, Michalopoulos GK. Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology. 1998;27:1030–1038.
Sheikh AY, Lin SA, Cao F, et al. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–2684.
Okada S, Nakauchi H, Nagayoshi K, et al. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050.
Perin EC, Dohmann HF, Borojevic R, et al. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110:II213–II218.
Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918.
Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148.
Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–3060.
Grompe M. The role of bone marrow stem cells in liver regeneration. Semin Liver Dis. 2003;23:363–372.
Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56:947–958.
Dekker A, Bulley S, Beyene J, et al. Meta-analysis of randomized controlled trials of prophylactic granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor after autologous and allogeneic stem cell transplantation. J Clin Oncol. 2006;24:5207–5215.
Romanienko PJ, Camerini-Otero RD. Cloning, characterization, and localization of mouse and human SPO11. Genomics. 1999;61:156–169.
Wexler SA, Donaldson C, Denning-Kendall P, et al. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374.
Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436.
Sugano Y, Anzai T, Yoshikawa T, et al. Granulocyte colony-stimulating factor attenuates early ventricular expansion after experimental myocardial infarction. Cardiovasc Res. 2005;65:446–456.
Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910.
Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638.
Gazitt Y, Akay C. Mobilization of myeloma cells involves SDF-1/CXCR4 signaling and downregulation of VLA-4. Stem Cells. 2004;22:65–73.
Acknowledgments
The authors would like to thank Dr. William Orr, Department of Pathology, University of Manitoba, Canada, for suggestions concerning the use of English in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, SZ., Meng, XW., Sun, X. et al. Granulocyte Colony-Stimulating Factor Enhances Bone Marrow Mononuclear Cell Homing to the Liver in a Mouse Model of Acute Hepatic Injury. Dig Dis Sci 55, 2805–2813 (2010). https://doi.org/10.1007/s10620-009-1117-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-1117-5