Abstract
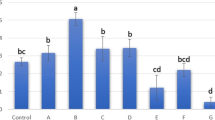
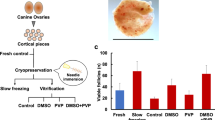
In this study, we investigated the temporal post-mortem limits, within which there will be guarantees of obtaining living cells from several tissues of sheep and cattle and the effect of vitrification on the ability of cells from tissue stored at different times. Muscle tissue and auricular cartilage were stored at 4°C for 5, 48, 72, 96 and 216 h post-mortem (hpm). Tissue samples were sorted into two groups: one group was in vitro cultured immediately after storage and the other was vitrified after storage and then in vitro cultured. In cattle and sheep, no differences in subconfluence rates were observed between the two experimental groups. At the same time, no significant differences were observed in the number of days required in culture to reach confluence between non-vitrified and vitrified groups when tissues were stored at 4°C for different times. In sheep, while the population doubling times (PDT) were similar in cartilage cells from vitrified and non-vitrified tissues and stored at 4°C for 5 and 216 hpm, PDT of muscle cells were longer in 216 hpm stored groups than in 5 hpm stored groups. In bovine, although the PDT of muscle cells were similar for 5 and 216 hpm and both vitrified and non-vitrified tissues and the PDT were longer in cartilage cells from vitrified than from non-vitrified tissues. In conclusion, although storage times and vitrification have different effects on tissues from cattle and sheep, this study showed that living cells could be obtained from all groups. Therefore, cartilage and muscle tissues can be stored at 4°C for 216 hpm and used for cyrobanking.


Similar content being viewed by others
References
Akkoc T, Taskin C, Caputcu TA, Arat S, Bagis H (2011) The effect of solid surface vitrificaiton (SSV) versus classic vitrification technique on survive rate of in vitro produced bovine blastocysts. J Anim Vet Adv 10(22):2885–2891
Andrabi SMH, Maxwell WMC (2007) A review of reproductive biotechnologies for conservation of endangered mammalian species. Anim Reprod Sci 99:223–243
Arat S, Gibbons J, Rzucidlo SJ, Respess DS, Tumlin M, Stice SL (2002) In vitro development of bovine nuclear transfer embryos from clonal lines of transgenic adult and fetal fibroblast cells of the same genotype. Biol Reprod 66:1768–1774
Arat S, Bagis H, Ergin F, Sagirkaya H, Mercan Odaman H, Dinnyes A (2004) Cold storage of tissue as source for donor cells does not reduce the in vitro development of bovine embryos following nuclear transfer. Reprod Fertil Dev 16(1,2):135
Arat S, Bagis H, Mercan Odaman H, Dinnyes A (2005) Cloned embryos can be produced using donor cells obtained from 72-hour cooled carcass. Reprod Fertil Dev 17:164
Arat S, Tas A, Akkoc T, Cetinkaya G, Bagis H, Sekmen S, Ates E, Soysal D (2009) Effect of growth factors on development of nuclear transfer embryos from cartilage cell of an Anatolian native cow. Reprod Domes Anim 44:93
Arat S, Caputcu AT, Akkoc T, Pabuccuoglu S, Sagirkaya H, Cirit U, Nak Y, Koban E, Bagis H, Demir K, Nak D, Senunver A, Kilicaslan R, Tuna B, Cetinkaya G, Denizci M, Aslan O (2011) Using cell banks as a tool in conservation programmes of native domestic breeds: the production of the first cloned Anatolian grey cattle. Reprod Fertil Dev 23(8):1012–1023
Asawa Y, Ogasawara T, Takahashi T, Yamaoka H, Nishizawa S, Matsudaira K, Mori Y, Takato T, Hoshi K (2009) Aptitude of auricular and nasoseptal chondrocytes cultured under a monolayer or three-dimensional condition for cartilage tissue engineering. Tissue Eng Part A 15:1109–1118
Bagis H, Akkoc T, Tas A, Aktoprakligil D (2008) Cryogenic effect of antifreeze protein on transgenic mouse ovaries and the production of live offspring by orthotopic transplantation of cryopreserved mouse ovaries. Mol Reprod Dev 75:608–613
Bagis H, Akkoc T, Taskin C, Arat S (2010) Comparison of different cryopreservation techniques: higher survival and implantation rate of frozen-thawed mouse pronuclear embryos in the presence of beta-mercaptoethanol in post-thaw culture. Reprod Domest Anim 45:332–337
Brem G, Kuhholzer B (2002) The recent history of somatic cloning in mammals. Cloning Stem Cells 4(1):57–63
Cetinkaya G, Arat S (2011) Cryopreservation of cartilage cell and tissue for biobanking. Cyrobiology 63:292–297
Day JG, Stacey GN (2007a) Cryopreservation and freeze-drying protocols. In: Day JG, Stacey GN (eds) Long-term ex situ conservation of biological resources and the role of biological resource centers, 2nd edn. Humana Press, New Jersey, pp 1–14
Day JG, Stacey GN (2007b) Cryopreservation and freeze-drying protocols. In: Sputtek A (ed) Cryopreservation of Red Blood Cells and Platelets, 2nd edn. Humana Press, New Jersey, pp 283–301
Fahy GM, Wowk B, Wu J, Phan J, Rasch C, Chang A, Zendejas E (2004) Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology 48:157–178
Fröhlich M, Malicev E, Gorensek M, Knezevic M, Velikonja NK (2007) Evaluation of rabbit auricular chondrocyte isolation and growth parameters in cell culture. Cell Biol Int 31:620–625
Gajda B, Katska-Ksiazkiewicz L, Rynska B, Bochenek M, Smorag Z (2007) Survival of bovine fibroblasts and cumulus cells after vitrification. Cryo Lett 28(4):271–279
Gañán N, Sestelo A, Garde JJ, Martínez F, Vargas A, Sánchez I, Pérez-Aspa MJ, López-Bao JV, Palomares F, Gomendio M, Roldan ER (2010) Reproductive traits in captive and free-ranging males of the critically endangered Iberian lynx (Lynx pardinus). Reproduction 139:275–285
Gibbons J, Arat S, Rzucidlo SJ, Waltenburg R, Respess DS, Venable AM, Stice SL (2002) Enhanced survivability of cloned calves derived from roscovitine-treated adult somatic cells. Biol Reprod 66:895–900
Hay RJ (1992) Cell line preservation and characterization. In: Freshney RI (ed) Animal Cell Culture. Oxford University Press, New York, A Practical Approach, pp 95–148
Ideta A, Hayama K, Urakawa M, Tsuchiya K, Aoyagi Y, Saeki K (2010) Comparison of early development in utero of cloned fetuses derived from bovine fetal fibroblasts at the G1 and G0/G1 phases. Anim Reprod Sci 119:191–197
Leon-Quinto T, Simon MA, Cadenas R, Jones J, Martinez-Hernandez FJ, Moreno JM, Vargas A, Martinez F, Soria B (2009) Developing biological resource banks as a supporting tool for wildlife reproduction and conservation the Iberian lynx bank as a model for other endangered species. Anim Reprod Sci 112:347–361
Loi P, Ptak G, Barboni B, Fulka J Jr, Cappai P, Clinton M (2001) Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat Biotech 19:962–964
Martin H, Bournique B, Sarsat JP, Albaladejo V, Lerche-Langand C (2000) Cryopreserved rat liver slices: a critical evaluation of cell viability, histological integrity, and drug metabolizing enzymes. Cryobiology 41:135–144
McLaren A (2000) Cloning: pathways to a pluripotent future. Science 288:1775–1780
Morgan SJ, Darling DC (1993) Animal cell culture, In: JM Graham, D Billington (eds), BIOS scientific publisher limited, UK, 161
Nel-Themaat L, Gómez MC, Damiani P, Wirtu G, Dresser BL, Bondioli KR, Lyons LA, Pope CE, Godke RA (2007) Isolation, culture and characterisation of somatic cells derived from semen and milk of endangered sheep and eland antelope. Reprod Fertil Dev 19:576–584
Orief Y, Schultze-Masgau A, Dafopoulus K, Al-hasani S (2005) Vitrification: will it replace the conventional gamete cryopreservation techniques. Middle East Fertil Soc J 10(3):171–184
Pegg DE, Wusteman MC, Wand L (2006) Cryopreservation of articular cartilage. Part 1: conventional cryopreservation methods. Cryobiology 52:335–346
Prentice JR, Anzar M (2011) Cryopreservation of mammalian oocyte for conservation of animal genetics. Vet Med Int. doi:10.4061/2011/146405
Ryder OA (2002) Cloning advances and challenges for conservation. Trends Biotechnol 20:231–232
Saeed AM, Escriba MJ, Silvestre MA, Garcia-Ximenez F (2000) Vitrification and rapid-freezing of cumulus cells from rabbits and pigs. Theriogenology 54:1359–1371
Shiga K, Fujita T, Hirose K, Sasae Y, Nagai T (1999) Production of calves by transfer of nuclei from cultured somatic cells obtained from Japanese black bulls. Theriogenology 52(3):527–535
Shroeder AC, Johnston D, Epping JJ (1991) Reversal of post-mortem degeneration of mouse oocytes during meiotic maturation in vitro. J Exp Zool 258:240–245
Silvestre MA, Saeed AM, Escriba MJ, Garcia-Ximenez F (2000) Vitrification and rapid-freezing of cumulus cells from rabbits and pigs. Theriogenology 54(9):1359–1371
Silvestre MA, Saeed AM, Escribá MJ, García-Ximénez F (2002) Vitrification and rapid freezing of rabbit fetal tissues and skin samples from rabbits and pigs. Theriogenology 58(1):69–76
Silvestre MA, Saeed AM, Cervera RP, Escriba MJ, Garcia-Ximenez F (2003) Rabbit and pig ear skin sample cryobanking: effects of storage time and temperature of the whole ear extirpated immediately after death. Theriogenology 59:1469–1477
Silvestre MA, Sanchez JP, Gomez EA (2004) Vitrification of goat, sheep, and cattle skin samples from whole ear extirpated after death and maintained at different storage times and temperatures. Cryobiology 49:221–229
Smith LC, Bordignon V, Babkine M, Fecteau G, Keefer C (2000) Benetif and problems with cloning animals. Can Vet J 4:919–924
Stolzing A, Scutt A (2006) Age-related impairment of mesenchymal progenitor cell function. Aging Cell 5:213–224
Vajta G, Nagy ZP, Cobo A, Conceicao J, Yovich J (2009) Vitrification in assisted reproduction: myths, mistakes, disbeliefs and confusion. Biomed Online 19:1–7
Wells DNA, Misica PM, Tervit HR, Vivanco WH (1998) Adult somatic cell NT in used to preserve the last surviving cow of Enderby Island cattle breed. Reprod Fertil Dev 10:369–378
Wildt DE, Wemmer C (1999) Sex and wildlife: the role of reproductive science in conservation. Biodivers Conserv 8:965–976
Wusteman M, Robinson M, Pegg D (2004) Vitrification of large tissues with dielectric warming: biological problems and some approaches to their solution. Cryobiology 48:179
Acknowledgments
We would like to thank Dr. Digdem Aktoprakligil Aksu for manuscript review; Fatih Karakaya, Erman Ates and Ozlem Celasin for technical assistance. The manuscript has been edited by native speaker Dr. Anita L. Akkas, who has PhD degree in English Literature and MA degree in Linguistics Engineering and Science. This research was funded by the Scientific and Technological Research Council of Turkey (TUBITAK) (Project no. KAMAG-106G005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caputcu, A.T., Akkoc, T., Cetinkaya, G. et al. Tissue cryobanking for conservation programs: effect of tissue type and storage time after death. Cell Tissue Bank 14, 1–10 (2013). https://doi.org/10.1007/s10561-012-9292-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-012-9292-6