Abstract
Objective
To study neutropenia hospitalization (NH) incidence and risk factors in a population-based sample of older adults with non-Hodgkin’s lymphoma (NHL) and evaluate the validity of inferences from Surveillance, Epidemiology and End Results (SEER)-Medicare linked databases.
Methods
NHL cases receiving first-course chemotherapy were identified from Iowa SEER-Medicare. Survival methods evaluated NH risk factors. Medical record and Medicare claims data on chemotherapy and NH were compared.
Results
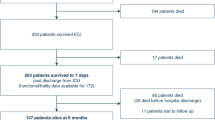
Of 761 subjects, 165 (21.7%, 95% CI: 18.8, 24.6) were hospitalized for neutropenia. Of those hospitalized, 41% were hospitalized in cycle 1 and 22% in cycle 2. Significant multivariable risk factors for NH were diffuse large cell histology, renal disease, Charlson comorbidity index, and anthracycline chemotherapy but not patient age. Medicare and medical records agreed on month of chemotherapy initiation 95% of the time and chemotherapy type 95% of the time. ICD-9 code 288.0 sensitivity for NH was 80%.
Conclusions
Neutropenia hospitalizations were common in the first 2 chemotherapy cycles, especially among older adults with comorbidity. Findings conflict with a prior medical records study in which age was a risk factor for NH and dose intensity a negative confounder. Valid inferences about age effects on chemotherapy toxicity require more clinical detail than is available in administrative data.




Similar content being viewed by others
References
Tejeda HA, Green SB, Trimble EL et al. (1996) Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst 88(12):812–816
Goodwin JS, Hunt WC, Humble CG, Key CR, Samet JM (1988) Cancer treatment protocols. Who gets chosen? Arch Intern Med 148(10):2258–2260
Trimble EL, Carter CL, Cain D, Freidlin B, Ungerleider RS, Friedman MA (1994) Representation of older patients in cancer treatment trials. Cancer 74(7 Suppl):2208–2214
Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr., Albain KS (1999) Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 341(27):2061–2067
Lewis JH, Kilgore ML, Goldman DP et al. (2003) Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 21(7):1383–1389
Murthy VH, Krumholz HM, Gross CP (2004) Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 291(22):2720–2726
Zippin C, Lum D, Hankey BF (1995) Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer 76:2343–2350
McClish DK, Penberthy L, Whittemore M et al. (1997) Ability of Medicare claims data and cancer registries to identify cancer cases and treatment. Am J Epidemiol 145:227–233
Brooks JM, Chrischilles E, Scott S, Ritho J, Chen-Hardee S (2000) Information gained from linking SEER Cancer Registry Data to state-level hospital discharge abstracts. Med Care 38:1131–1140
Doebbeling BN, Wyant DK, McCoy KD et al. (1999) Linked insurance-tumor registry database for health services research. Med Care 37:1105–1115
Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG (1993) Potential for cancer-related health services research using a linked Medicare-tumor registry database. Med Care 31:732–748
Chrischilles E, Link B, Scott S, Delgado D, Fridman M (2003) Factors associated with early termination of CHOP therapy and the impact on survival among patients with chemosensitive intermediate-grade non-Hodgkin’s lymphoma. Cancer Control 10:396–403
Warren JL, Harlan LC, Fahey A et al. (2002) Utility of the SEER-Medicare data to identify chemotherapy use. Med Care 40(suppl 4):55–61
Du X, Goodwin JS (2001) Patterns of use of chemotherapy for breast cancer in older women: Findings from Medicare claims data. J Clin Oncol 19:1455–1461
Lamont EB, Lauderdale DS, Schilsky RL, Christakis NA (2002) Construct validity of Medicare chemotherapy claims. The case of 5FU. Med Care 40:201–211
Du XL, Osborne C, Goodwin JS (2002) Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol 20:4636–4642
Ries LAG, Eisner MP, Kosary CL et al. (eds) (2002) SEER Cancer Statistics Review, 1973–1999. Bethesda, MD: National Cancer Institute, http://seer.cancer.gov/csr/1973_1999/
Annon (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors project. N Engl J Med 329:987–994
Solal-Celigny P. Roy P., Colombat P et al. (2004) Follicular lymphoma international prognostic index. Blood 104(5):1258–1265
Tirelli U, Errante D, Van Glabbeke M et al. (1998) CHOP is the standard regimen in patients ≥70 years of age with intermediate-grade and high-grade non-Hodgkin’s lymphoma: results of a randomized study of the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Study Group. J Clin Oncol 16:27–34
Sonneveld P, de Ridder M, van der Lelie H et al. (1995) Comparison of doxorubicin and mitoxantrone in the treatment of elderly patients with advanced diffuse non-Hodgkin’s lymphoma using CHOP versus CNOP chemotherapy. J Clin Oncol 13:2530–2539
Meyer RM, Browman GP, Samosh ML et al. (1995) Randomized phase II comparison of standard CHOP with weekly CHOP in elderly patients with non-Hodgkin’s lymphoma. J Clin Oncol 13:2386–2393
Lyman GH, Morrison VA, Dale DC, Crawford J, Delgado DJ, Fridman M (2003) Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 44:2069–2076
Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG (1993) Potential for cancer-related health services research using a linked Medicare tumor registry database. Med Care 31:732–748
Clinical Classifications Software (ICD-9-CM) Summary and Download. Summary and Downloading Information. April 2003. Agency for Health Care Policy and Research, Rockville, MD, http://www.ahrq.gov/data/hcup/ccs.htm
Deyo RA, Cherkin C, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619
Morrison VA, Picozzi V, Scott S et al. (2001) The impact of age on delivered dose intensity and hospitalizations for febrile neutropenia in patients with intermediate-grade non-Hodgkin’s lymphoma receiving initial CHOP chemotherapy: A risk factor analysis. Clin Lymphoma 2:47–56
Picozzi VJ, Pohlman BL, Morrison VA et al. (2001) Patterns of chemotherapy administration in patients with intermediate-grade non-Hodgkin’s lymphoma. Oncology 15:1296–1306
Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI (2004) Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: A nationwide study. J Clin Oncol 22:4302–4311
Voelker MD, Chrischilles E, Wright K, Link BK, Park T, Delgado D (2004) Factors associated with first course chemotherapy among older patients with newly diagnosed non-Hodgkin’s lymphoma: National SEER-Medicare study. J Clin Oncol 22(14S: Abstract 6118)
Acknowledgements
This study was funded by an unrestricted research grant from Amgen, Inc.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen-Hardee, S., Chrischilles, E.A., Voelker, M.D. et al. Population-based Assessment of Hospitalizations for Neutropenia from Chemotherapy in Older Adults with Non-Hodgkin’s Lymphoma (United States). Cancer Causes Control 17, 647–654 (2006). https://doi.org/10.1007/s10552-005-0502-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10552-005-0502-4