Abstract
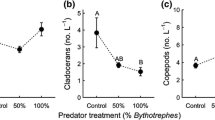
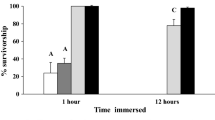
Invasive predators can have large negative effects on native prey populations. The susceptibility of native prey to invasive predators may depend on their ability to respond behaviorally to the presence of these non-native predators. In a field survey conducted in Lake Michigan over several years, we found that high densities of the invasive predatory cladoceran Bythotrephes were correlated with lower vertical distributions of some species and age classes of native copepods; moving from inhabiting primarily the epiliminion at low Bythotrephes density to primarily the hypolimnion at high Bythotrephes density. Five groups showed this pattern; diaptomid copepodites, adult cyclopoids, Diacyclops thomasi, and the adult diaptomids Leptodiaptomus ashlandi and L. minutus. In contrast, Bythotrephes density was not correlated with the vertical distribution of copepod nauplii and adult L. sicilis. Laboratory experiments suggest that the changes in the vertical distribution in the field at high Bythothrephes are due to an inducible, plastic response to predation threat from Bythotrephes signaled by water-borne cues. Species that were lower in the field at high Bythotrephes densities responded behaviorally to water-borne cues from Bythotrephes by moving to lower levels of experimental water columns. These species included D. thomasi and L. minutus, with L. ashlandi displaying a non-significant trend in the same direction. In contrast, L. sicilis, which was not correlated with Bythotrephes density in the field, was unaffected by the water-borne cues. Differences in vertical distribution shifts among these native copepod species and life-history stages are likely due to species-specific differences in spatial overlap with Bythotrephes and their relative ability to migrate large distances or employ alternative avoidance strategies. The varied responses exhibited among the copepod groups likely alter their interactions with each other, their resources and other predators, thus revealing the complex effects Bythotrephes can have on invaded communities.



Similar content being viewed by others
References
Abrams PA (1992) Predators that benefit prey and prey that harm predator—unusual effects of interacting foraging adaptations. Am Nat 140:573–600
Balcer MD, Korda NL, Dodson SI (1984) Zooplankton of the great lakes: a guide to the identification and ecology of the common crustacean species. The University of Wisconsin Press, Madison
Blumstein DT, Daniel JC, Springett BP (2004) A test of the multi-predator hypothesis: rapid loss of antipredator behavior after 130 years of isolation. Ethol 110:919–934
Brandt SB, Mason DM, Patrick EV, Argyle RL, Wells L, Unger PA, Stewart DJ (1991) Acoustic measures of the abundance and size of pelagic planktivores in Lake Michigan. Can J Fish Aquat Sci 48:894–908
Bremigan MT, Dettmers JM, Mahan AL (2003) Zooplankton selectivity by larval yellow perch in Green Bay, Lake Michigan. J Great Lakes Res 29:501–510
Carrick HJ, Fahnenstiel GL, Stoermer EF, Wetzel RG (1991) The importance of zooplankton-protozoan trophic coupling in Lake Michigan. Limnol Oceanogr 36:1335–1345
Castel J, Veiga J (1990) Distribution and retention of the copepod Eurytemora affinis hirundoides in a turbid estuary. Mar Biol 107:119–128
Cieri MD, Stearns DE (1999) Reduction of grazing activity of two estuarine copepods in response to the exudate of a visual predator. Mar Ecol Prog Ser 177:157–163
Clavero M, Garcia-Berthou E (2005) Invasive species are a leading cause of animal extinctions. Trends Ecol Evol 20:110
Cohen JH, Forward RB (2009) Zooplankton diel vertical migration—a review of proximate control. Oceanogr Mar Biol Annu Rev 47:77–109
Cox JG, Lima SL (2006) Naivete and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680
De Meester L, Dawidowics P, Gool E, Loose C (1999) Ecology and evolution of predator behavior in zooplankton: depth selection behavior and diel vertical migration. In: Tollrian R, Harvell C (eds) The ecology and evolution of inducible defenses, Chap 9. Princeton University Press, Princeton, pp 160
DeWitt TJ, Sih A, Hucko JA (1999) Trait compensation and cospecialization in a freshwater snail: size, shape and antipredator behaviour. Anim Behav 58:397–407
Estes JA, Palmisan JF (1974) Sea otters—their role in structuring nearshore communities. Science 185:1058–1060
Gerritsen J (1978) Instar-specific swimming patterns and predation of planktonic copepods. Proceedings. Congress in Denmark 1977, Internationale Vereinigung fur Theoretische und Angewandte Limnologie, vol 20, Part 4, pp 2531–2536. 4 Fig, 1 Tab, 17 Ref. NSF DEB 76-02096
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends Ecol Evol 19:470–474
Hoffman JC, Smith ME, Lehman JT (2001) Perch or plankton: top-down control of Daphnia by yellow perch (Perca flavescens) or Bythotrephes cederstroemi in an inland lake? Freshw Biol 46:759–775
Kats LB, Ferrer RP (2003) Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers Distrib 9:99–110
Landry MR (1978) Population dynamics and production of a planktonic marine copepod, Acartia clausii, in a small temperate lagoon on San Juan Island, Washington. Int Rev Gesamten Hydrobiol 63:77–119
Lass S, Spaak P (2003) Chemically induced anti-predator defences in plankton: a review. Hydrobiologia 491:221–239
Liebig JR, Vanderploeg HA (1995) Vulnerability of Dreissena polymorpha larvae to predation by Great Lakes calanoid copepods: the imporance of the bivalve shell. J Great Lakes Res 21:353–358
Loose CJ, Dawidowicz P (1994) Trade-offs in diel vertical migration by zooplankton—costs of predator avoidance. Ecology 75:2255–2263
Loose CJ, von Elert E, Dawidowicz P (1993) Chemically-induced diel vertical migration in Daphnia—a new bioassay from kairomones exuded by fish. Archiv fur Hydrobiologie 126:329–337
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Miller T, Crowder LB, Binkowksi FP (1990) Effects of changes in the zooplankton assemblage on growth of bloater and implications for recruitment success. Trans Am Fish Soc 119:483–491
Pangle KL, Peacor SD (2006) Non-lethal effect of the invasive predator Bythotrephes longimanus on Daphnia mendotae. Freshw Biol 51:1070–1078
Pangle KL, Peacor SD (2009) Light-dependent predation by the invertebrate planktivore Bythotrephes longimanus. Can J Fish Aquat Sci 66:1748–1757
Pangle KL, Peacor SD (2010) Temperature gradients, not food resource gradients, affect growth rate of migrating Daphnia mendotae in Lake Michigan. J Great Lakes Res 36:345–350
Pangle KL, Peacor SD, Johannsson OE (2007) Large nonlethal effects of an invasive invertebrate predator on zooplankton population growth rate. Ecology 88:402–412
Peacor SD, Werner EE (2000) Predator effects on an assemblage of consumers through induced changes in consumer foraging behavior. Ecology 81:1998–2010
Peacor SD, Werner EE (2001) The contribution of trait-mediated indirect effects to the net effects of a predator. Proc Natl Acad Sci U S A 98:3904–3908
Pennak RW (1978) Fresh-water invertebrates of the United States. Wiley-Interscience Publication, New York
Pichlová-Ptáčníková R, Vanderploeg HA (2011) The quick and the dead: might differences in escape rates explain the changes in the zooplankton community composition of Lake Michigan after invasion by Bythotrephes? Biol Invasions. doi:10.1007/s10530-011-0076-x
Pijanowska J (1997) Alarm signals in Daphnia? Oecologia 112:12–16
Power ME (1990) Effects of fish in river food webs. Science 250:811–814
Preisser EL, Bolnick DI (2008) The many faces of fear: comparing the pathways and impacts of nonconsumptive predator effects on prey populations. Plos One 3:e2465
Preisser EL, Bolnick DI, Benard MF (2005) Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86:501–509
Ramcharan CW, Sprules WG (1991) Predator-induced behavioral defense and its ecological consequences for 2 calanoid copepods. Oecologia 86:276–286
Relyea RA (2000) Trait-mediated indirect effects in larval anurans: reversing competition with the threat of predation. Ecology 81:2278–2289
Roff JC, Hopcroft RR (1986) High-precision microcomputer based measuring system for ecological research. Can Fish Aquat Sci 63:2878–2881
Roman MR, Holliday DV, Sanford LP (2001) Temporal and spatial patterns of zooplankton in the Chesapeake Bay turbidity maximum. Mar Ecol Prog Ser 213:215–227
Salo P, Korpimaki E, Banks PB, Nordstrom M, Dickman CR (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc B Biol Sci 274:1237–1243
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator-prey naivete, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Soule ME (1990) The onslaught of alien species, and other challenges in the coming decades. Conserv Biol 4:233–239
Torke B (2001) The distribution of calanoid copepods in the plankton of Wisconsin lakes. Hydrobiologia 453(454):351–365
Trussell GC, Ewanchuk PJ, Bertness MD (2002) Field evidence of trait-mediated indirect interactions in a rocky intertidal food web. Ecol Lett 5:241–245
Turner AM, Bernot RJ, Boes CM (2000) Chemical cues modify species interactions: the ecological consequences of predator avoidance by freshwater snails. Oikos 88:148–158
Ueda H (1987) Small-scale ontogenetic and diel vertical distributions of neritic copepods in Maizuru Bay, Japan. Mar Ecol Prog Ser 35:65–73
van Gool E, Ringelberg J (1998) Light-induced migration behaviour of Daphnia modified by food and predator kairamones. Anim Behav 56:741–747
Vanderploeg HA, Liebig JR, Omair M (1993) Bythotrephes predation on Great Lakes zooplankton measured by an in situ method—implications for zooplankton community structure. Archiv fur Hydrobiologie 127:1–8
Vitousek PM, Dantonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am Sci 84:468–478
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth’s ecosystems. Science 277:494–499
Warren GJ (1983) Predation by Limnocalanus as a source of winter nauplier mortality in Lake Michigan. J Great Lakes Res 9:389–395
Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100
Yan ND, Pawson TW (1998) Seasonal variation in the size and abundance of the invading Bythotrephes in Harp lake, Ontario, Canada. Hydrobiol 361:157–168
Acknowledgments
We would like to thank Dennis Donahue for logistical support and the crew of the R. V. Laurentian for their help with the field surveys. Anne Kellerman, Rachel Komosinski, Damon Kreuger, Emily Reed and Alex Sookhai assisted with field and laboratory work. Comments by two anonymous reviewers improved this manuscript. Funding for this study was provided by the Fishery Research Program of the Great Lakes Fisheries Commission, the National Oceanic and Atmospheric Administration, and National Science Foundation grants OCE-0826020 to SDP.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Fig. 4.
Relationship between fish abundance (log[number of fish/hectare]) and Bythotrephes abundance (log[mg/m2]) from M45, M60, M65, and M110 stations (corresponding to 45, 60, 65 and 110 m depths) in Lake Michigan near Muskegon, MI from July–August 2004. Fish data derived from acoustic sampling (D. Krueger, unpublished data)
Rights and permissions
About this article
Cite this article
Bourdeau, P.E., Pangle, K.L. & Peacor, S.D. The invasive predator Bythotrephes induces changes in the vertical distribution of native copepods in Lake Michigan. Biol Invasions 13, 2533–2545 (2011). https://doi.org/10.1007/s10530-011-0073-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0073-0