Abstract
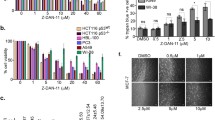
Shogaols have been previously reported to induce cancer cell death via multiple mechanisms, among which one analog 6-shogaol has been reported to cause microtubule damage through specific reaction with sulfhydryl groups in tubulin. In this study, a series of shogaols with different side chain lengths (4-, 6-, 8- and 10-shogaol) was synthesized and evaluated for antiproliferative activity in HCT 116 colon carcinoma and SH-SY5Y neuroblastoma cells. 4- and 6-shogaol were identified as lead compounds possessing the strongest antiproliferative activity. In the soft agar assay, the lead shogaols displayed dose-dependent inhibition on cancer cell colony formation under anchorage-independent conditions. Using HCT 116 as the selected cancer cell line, the molecular events linking shogaols-induced G2/M cell cycle arrest to apoptosis characterized by caspase 3 and PARP cleavage were investigated. At sublethal concentrations, the halt at G2/M phase was alleviated along time and cells survived. Conversely, proapoptotic concentrations of 4- and 6-shogaol induced irreversible G2/M arrest that was at least in part associated with down-regulation of cell cycle checkpoint proteins cdk1, cyclin B and cdc25C, as well as spindle assembly checkpoint proteins mad2, cdc20 and survivin. A dose- and time-dependent accumulation of insoluble tubulin in the insoluble fractions of cell lysates provided evidence that G2 checkpoint failure led to disruption of microtubule turnover. In summary, our results conclude that shogaols cause apoptosis by inducing aberrant mitosis at least through the attenuation of cell cycle and spindle assembly checkpoint proteins.





Similar content being viewed by others
Abbreviations
- CAK:
-
Cdk activating kinase
- Cdk:
-
Cyclin-dependent kinase
- mad2:
-
Mitotic-arrest deficient homologue-2
- p-cdk1:
-
Phospho cyclin-dependent kinase 1
References
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Singh P, Rathinasamy K, Mohan R, Panda D (2008) Microtubule assembly dynamics: an attractive target for anticancer drugs. IUBMB Life 60:368–375
Sherr CJ (1996) Cancer cell cycles. Science 274:1672–1677
Schwartz GK, Shah MA (2005) Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol 23:9408–9421
Johansson M, Persson JL (2008) Cancer therapy: targeting cell cycle regulators. Anticancer Agents Med Chem 8:723–731
Ali BH, Blunden G, Tanira MO, Nemmar A (2008) Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol 46:409–420
Ma J, Jin X, Yang L, Liu ZL (2004) Diarylheptanoids from the rhizomes of Zingiber officinale. Phytochemistry 65:1137–1143
Wei QY, Ma JP, Cai YJ, Yang L, Liu ZL (2005) Cytotoxic and apoptotic activities of diarylheptanoids and gingerol-related compounds from the rhizome of Chinese ginger. J Ethnopharmacol 102:177–184
Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN (2004) Fresh organically grown ginger (Zingiber officinale): composition and effects on LPS-induced PGE2 production. Phytochemistry 65:1937–1954
Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN (2005) Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE2 production. Phytochemistry 66:1614–1635
Lee S, Khoo C, Halstead CW, Huynh T, Bensoussan A (2007) Liquid chromatographic determination of 6-, 8-, 10-gingerol, and 6-shogaol in ginger (Zingiber officinale) as the raw herb and dried aqueous extract. J AOAC Int 90:1219–1226
Pan MH, Hsieh MC, Hsu PC, Ho SY, Lai CS, Wu H, Sang S, Ho CT (2008) 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol Nutr Food Res 52:1467–1477
Levy AS, Simon O, Shelly J, Gardener M (2006) 6-Shogaol reduced chronic inflammatory response in the knees of rats treated with complete Freund’s adjuvant. BMC Pharmacol 6:12
Ling H, Yang H, Tan SH, Chui WK, Chew EH (2010) 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-κB activation. Br J Pharmacol 161:1763–1777
Weng CJ, Wu CF, Huang HW, Ho CT, Yen GC (2010) Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol Nutr Food Res 54:1618–1627
Chen CY, Liu TZ, Liu YW, Tseng WC, Liu RH, Lu FJ, Lin YS, Kuo SH, Chen CH (2007) 6-Shogaol (alkanone from ginger) induces apoptotic cell death of human hepatoma p53 mutant Mahlavu subline via an oxidative stress-mediated caspase-dependent mechanism. J Agric Food Chem 55:948–954
Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H, Sang S, Ho CT (2008) 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol Nutr Food Res 52:527–537
Shieh PC, Chen YO, Kuo DH, Chen FA, Tsai ML, Chang IS, Wu H, Sang S, Ho CT, Pan MH (2010) Induction of apoptosis by [8]-shogaol via reactive oxygen species generation, glutathione depletion, and caspase activation in human leukemia cells. J Agric Food Chem 58:3847–3854
Ishiguro K, Ando T, Watanabe O, Goto H (2008) Specific reaction of alpha, beta-unsaturated carbonyl compounds such as 6-shogaol with sulfhydryl groups in tubulin leading to microtubule damage. FEBS Lett 582:3531–3536
Denniff P, Macleod I, Whiting DA (1981) Syntheses of the (±)-[N]-gingerols (pungent principles of ginger) and related-compounds through regioselective aldol condensations—relative pungency assays. J Chem Soc 1:82–87
Kim DS, Kim JY (2004) Side-chain length is important for shogaols in protecting neuronal cells from beta-amyloid insult. Bioorg Med Chem Lett 14:1287–1289
Mi L, Gan N, Cheema A, Dakshanamurthy S, Wang X, Yang DC, Chung FL (2009) Cancer preventive isothiocyanates induce selective degradation of cellular alpha- and beta-tubulins by proteasomes. J Biol Chem 284:17039–17051
Sang S, Hong J, Wu H, Liu J, Yang CS, Pan MH, Badmaev V, Ho CT (2009) Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J Agric Food Chem 57:10645–10650
Kim JS, Lee SI, Park HW, Yang JH, Shin TY, Kim YC, Baek NI, Kim SH, Choi SU, Kwon BM, Leem KH, Jung MY, Kim DK (2008) Cytotoxic components from the dried rhizomes of Zingiber officinale Roscoe. Arch Pharm Res 31:415–418
Castedo M, Perfettini JL, Roumier T, Kroemer G (2002) Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ 9:1287–1293
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9:153–166
Nigg EA (2001) Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2:21–32
Smits VA, Medema RH (2001) Checking out the G(2)/M transition. Biochim Biophys Acta 1519:1–12
O’Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC (2000) Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA 97:13103–13107
Fourest-Lieuvin A, Peris L, Gache V, Garcia-Saez I, Juillan-Binard C, Lantez V, Job D (2006) Microtubule regulation in mitosis: tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Mol Biol Cell 17:1041–1050
Tsukahara T, Tanno Y, Watanabe Y (2010) Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature 467:719–723
Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8:379–393
Altieri DC (2003) Survivin in apoptosis control and cell cycle regulation in cancer. Prog Cell Cycle Res 5:447–452
Mita AC, Mita MM, Nawrocki ST, Giles FJ (2008) Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 14:5000–5005
Saxton WM, Stemple DL, Leslie RJ, Salmon ED, Zavortink M, McIntosh JR (1984) Tubulin dynamics in cultured mammalian cells. J Cell Biol 99:2175–2186
Salmon ED, Leslie RJ, Saxton WM, Karow ML, McIntosh JR (1984) Spindle microtubule dynamics in sea urchin embryos: analysis using a fluorescein-labeled tubulin and measurements of fluorescence redistribution after laser photobleaching. J Cell Biol 99:2165–2174
Acknowledgments
This work was supported by National University of Singapore Academic Research Fund Tier 1 R-148-000-116-112 and National Medical Research Council Grant No. NMRC/NIG/0050/2009 (E–H. Chew).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiaohui Ang, Olivia Huixian Ho and Sock-Hoon Tan contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gan, FF., Nagle, A.A., Ang, X. et al. Shogaols at proapoptotic concentrations induce G2/M arrest and aberrant mitotic cell death associated with tubulin aggregation. Apoptosis 16, 856–867 (2011). https://doi.org/10.1007/s10495-011-0611-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-011-0611-3