Abstract
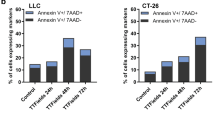
NMR technology has dramatically contributed to the revolution of image diagnostic. NMR apparatuses use combinations of microwaves over a homogeneous strong (1 Tesla) static magnetic field. We had previously shown that low intensity (0.3–66 mT) static magnetic fields deeply affect apoptosis in a Ca2+ dependent fashion (Fanelli et al., 1999 FASEBJ., 13;95–102). The rationale of the present study is to examine whether exposure to the static magnetic fields of NMR can affect apoptosis induced on reporter tumor cells of haematopoietic origin. The impressive result was the strong increase (1.8–2.5 fold) of damage-induced apoptosis by NMR. This potentiation is due to cytosolic Ca2+ overload consequent to NMR-promoted Ca2+ influx, since it is prevented by intracellular (BAPTA-AM) and extracellular (EGTA) Ca2+ chelation or by inhibition of plasma membrane L-type Ca2+ channels. Three-days follow up of treated cultures shows that NMR decrease long term cell survival, thus increasing the efficiency of cytocidal treatments. Importantly, mononuclear white blood cells are not sensitised to apoptosis by NMR, showing that NMR may increase the differential cytotoxicity of antitumor drugs on tumor vs normal cells. This strong, differential potentiating effect of NMR on tumor cell apoptosis may have important implications, being in fact a possible adjuvant for antitumor therapies.
Similar content being viewed by others
References
Feychting M, Ahlbom A. Magnetic fields leukaemia and central nervous system tumours in Swedish adults resident near high voltage power lynes. Epidemiology 1994; 5: 501–509.
Kheifets LI. Electric and magnetic field exposure and brain cancer: A review. Bioelectromagnetics 2001; 5: S120.
Cantoni O, Sestili P, Fiorani M, Dacha M. Effect of 50 Hz sinusoidal electric and/or magnetic fields on the rate of repair of DNA single strand breacks in cultured mammalian cells exposed to three different carginogens: methylmetha ne sulphonate, chromate and 254 nm UV radiation. Biochem Mol Biol Int 1996; 38: 527–533.
Fiorani M, Cantoni M, Sestili P, et al. Electric and/or magnetic field effect on DNA structure and function in cultured human cells. Mutat Res 1992; 282: 25–29.
Lacy-Hulbert A, Metcalfe JC, Hesketh R. Biological Responses to Magnetic Fields. FASEB J 1998; 12: 395–420.
Aldinucci C, Garcia JB, Palmi M, et al. The effect of Strong, Static Magnetic, Field on Lymphocytes. Bioelectromagnetics 2003; 24: 109–117.
Aldinucci C, Garcia JB, Palmi M, et al. The effect of exposure to high flux density static and pulsed magnetic field on lymphocytes function. Bioelectromagnetics 2003; 24: 373–379.
Paradisi S, Donelli G, Santini MT, Straface E, Malorni W. A 50-Hz magnetic field induces structural and biophysical changes in membranes. Bioelectromagnetics 1993; 14: 247–255.
Walleczek J, and Liburdy RP. Nonthermal 60 Hz sinusoidal magnetic field exposure enhance Ca2+ uptake in rat thymocytes: Dependence on mitogen activation. FEBS Lett. 1990; 271: 157–160.
Liburdy RP, Callahan DE, Harland J, Dunham E, Sloma TR, Yaswen P. Experimental evidence for 60 Hz magnetic fields operating through the signal transduction cascade: Effects on calcium influx and c-myc mRNA induction. FEBS Lett 1993; 334: 301–308.
Flipo D, Fournier M, Benquet C, et al. Increased apoptosis, changes in intracellular Ca2+, and functional alterations in lymphocytes and macrophages after in vitro exposure to static magnetic field. J Toxicol Environ Health A 1998; 54: 63–76.
Liboff AR. Cyclotron resonance in membrane transport. In A. Chiabrera et al., eds. Interactions between electromagnetic fields and cells. Plenum, New York, 1985: 281–296.
Fanelli C, Coppola S, Barone R, et al. Magnetic, Fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J 1999; 13: 95–102.
Teodori L, Gohde W, Valente MG, et al. Static magnetic fields affect calcium fluxes and inhibit stress-induced apoptosis in human glioblastoma cells. Cytometry 2002; 49: 113–118.
Dini L, Coppola S, Ruzittu MT, Ghibelli L. Multiple pathways for apoptotic nuclear fragmentation. Exp Cell Res 1996; 223: 340–347.
Ghibelli L, Maresca V, Coppola S, Gualandi G. Protease inhibitors block apoptosis at intermediate stages: A compared analysis of DNA fragmentation and apoptotic nuclear morphology. FEBS Lett 1995; 377: 9–14.
Ghibelli L, Fanelli C, Rotilio G, et al. Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J 1998; 12: 479–486.
Ghibelli L, Coppola S, Fanelli C, et al. Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. FASEB J 1999; 13: 2031–2036.
Nosseri C, Coppola S, Ghibelli L. Possible involvement of Poly(ADP-Ribosyl) Polymerase in triggering stress-induced apoptosis. Exp Cell Res 1994; 212: 367–373.
Coppola S, Nosseri C, Maresca V, Ghibelli L. Different basal NAD levels determine opposite effects of Poly(ADP-Ribosyl)Polimerase Inhibitors on H2O2-Induced apoptosis. Exp Cell Res 1995; 221: 462–469.
Ghibelli L, Nosseri C, Coppola S, Maresca V, Dini L. The increase in H2O2-induced apoptosis by ADP-ribosylation inhibitors is related to cell blebbing. Exp Cell Res 1995; 221: 470–477.
Colussi C, Albertini MC, Coppola S, Rovidati S, Galli F, Ghibelli L. H2O2-induced block of glycolysis as an active ADP-ribosylation reaction protecting cells from apoptosis. FASEB J 2000; 14: 2266–2276.
D'Alessio M, Cerella C, Amici C, et al. Glutathione depletion up-regulates Bcl-2 in BSO resistant-cells. FASEB J. 2004; 18: 1609–1611.
Ding GR, Nakahara T, Tian FR, Guo Y, Miyakoshi J. Transient suppression of X-ray-induced apoptosis by exposure to power frequency magnetic fields in MCF-7 cells.Biochem Biophys Res Commun 2001; 286: 953–957.
Tian F, Nakahara T, Yoshida M, Honda N, Hirose H, Miyakoshi J. Exposure to power frequency magnetic fields suppresses X-ray induced apoptosis transiently in Ku80-deficient xrs5 cells. Biophys Res Commun 2002; 292: 355–361.
Breckenridge DJ, Germain M, Mathai JP, Nguyen M, Shore GC, Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 2003; 22: 8608–8618.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghibelli, L., Cerella, C., Cordisco, S. et al. NMR exposure sensitizes tumor cells to apoptosis. Apoptosis 11, 359–365 (2006). https://doi.org/10.1007/s10495-006-4001-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-4001-1