Abstract
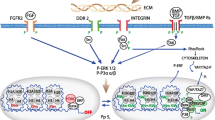
Growth plate and long bone development is governed by biochemical signaling pathways of which the PTHrP–Ihh system is the best known. Other factors, such as BMPs, FGFs and mechanical loading, may interact with this system. This study aims at elucidating the relative importance of PTHrP and Ihh for controlling proliferation, and hypertrophy in fetal growth plate cartilage. We assessed the question why reduced Ihh expression leads to more pronounced effects on the number of non-hypertrophic cells and total bone formation, compared to PTHrP down-regulation.
Using few basic equations, constituted from literature data, this paper shows how the PTHrP–Ihh feedback system can control different aspects of tissue differentiation at distinct locations. In particular, it is shown that (mechanical or biochemical) perturbations will affect proliferation via Ihh-related parameters, whereas changes in PTHrP-related parameters selectively interact with hypertrophy. This is contra-intuitive, since PTHrP acts to keep cells proliferating. In this context, the critical PTHrP level for keeping cells proliferating has been reconsidered. In addition, an explanation is provided for the aforementioned difference in effect between reduced Ihh and PTHrP expression.
Similar content being viewed by others
References
Amizuka N, Davidson D, Liu H, Valverde-Franco G, Chai S, Maeda T, Ozawa H, Hammond V, Ornitz DM, Goltzman et al. (2004) Signalling by fibroblast growth factor receptor 3 and parathyroid hormone-related peptide coordinate cartilage and bone development. Bone 34:13–25
Bailon-Plaza A, van der Meulen MC (2001) A mathematical framework to study the effects of growth factor influences on fracture healing. J Theor Biol 212:191–209
Casali A, Struhl G (2004) Reading the Hedgehog morphogen gradient by measuring the ratio of bound to unbound Patched protein. Nature 431:76–80
Chung UI, Lanske B, Lee K, Li E, Kronenberg H (1998) The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci USA 95:13030–13035
D’Angelo M, Sarment DP, Billings PC, Pacifici M (2001) Activation of transforming growth factor in chondrocytes undergoing endochondral ossification. J Bone Miner Res 16:2339–2347
Edelman ER, Nugent MA, Karnovsky MJ (1993) Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proc Natl Acad Sci USA 90:1513–1517
van’t Veen SJ, Hagen JW, van Ginkel FC, Prahl-Andersen B, Burger EH (1995) Intermittent compression stimulates cartilage mineralization. Bone 17:461–465
van der Eerden BC, Karperien M, Gevers EF, Lowik CW, Wit JM (2000) Expression of Indian hedgehog, parathyroid hormone-related protein, and their receptors in the postnatal growth plate of the rat: evidence for a locally acting growth restraining feedback loop after birth. J Bone Miner Res 15:1045–1055
Garcia AM, Frank EH, Grimshaw PE, Grodzinsky AJ (1996) Contributions of fluid convection and electrical migration to transport in cartilage: relevance to loading. Arch Biochem Biophys 333:317–325
Karp SJ, Schipani E, St Jacques B, Hunzelman J, Kronenberg H, McMahon AP (2000) Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development 127:543–548
Karperien M, Lanser P, De Laat SW, Boonstra J, Defize LHK (1996) Parathyroid hormone related peptide mRNA expression during murine postimplantation development: evidence for involvement in multiple differentiation processes. Int J Dev Biol 40:599–608
Klein-Nulend J, Veldhuijzen JP, Burger EH (1986) Increased calcification of growth plate cartilage as a result of compressive force in vitro. Arthritis Rheum 29:1002–1009
Klein-Nulend J, Veldhuijzen JP, van de Stadt RJ, van Kampen GP, Kuijer R, Burger EH (1987) Influence of intermittent compressive force on proteoglycan content in calcifying growth plate cartilage in vitro. J Biol Chem 262:15490–15495
Kobayashi T, Chung UI, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM (2002) PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development 129:2977–2986
Koziel L, Kunath M, Kelly OG, Vortkamp A (2004) Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev Cell 6:801–813
Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423:332–336
Leddy HA, Guilak F (2003) Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann Biomed Eng 31:753–760
Lee K, Lanske B, Karaplis AC, Deeds JD, Kohno H, Nissenson RA, Kronenberg HM, Segre GV (1996) Parathyroid hormone-related peptide delays terminal of chondrocytes during endochondral bone development. Endocrinology 137:5109–5118
Lerner AL, Kuhn JL, Hollister SJ (1998) Are regional variations in bone growth related to mechanical stress and strain parameters?. J Biomech 31:327–335
Long F, Linsenmayer TF (1998) Regulation of growth region cartilage proliferation and differentiation by perichondrium. Development 125:1067–1073
Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A (2002) Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell 3:439–449
Mosley JR, Lanyon LE (2002) Growth rate rather than gender determines the size of the adaptive response of the growing skeleton to mechanical strain. Bone 30:314–319
Nakamata T, Aoyama T, Okamoto T, Hosaka T, Nishijo K, Nakayama T, Nakamura T, Toguchida J (2003) In vitro demonstration of cell-to-cell interaction in growth plate cartilage using chondrocytes established from p53-/- mice. J Bone Miner Res 18:97–107
Naski MC, Ornitz DM (1998) FGF signaling in skeletal development. Front Biosci 3:d781–d794
Nimer E, Schneiderman R, Maroudas A (2003) Diffusion and partition of solutes in cartilage under static load. Biophys Chem 106:125–146
Ohashi N, Robling AG, Burr DB, Turner CH (2002) The effects of dynamic axial loading on the rat growth plate. J Bone Miner Res 17:284–292
Ornitz DM, Marie PJ (2002) FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev 16:1446–1465
Pathi S, Pagan-Westphal S, Baker DP, Garber EA, Rayhorn P, Bumcrot D, Tabin CJ, Blake Pepinsky R, Williams KP (2001) Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech Dev 106:107–117
Quinn TM, Kocian P, Meister JJ (2000) Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch Biochem Biophys 384:327–334
Quinn TM, Morel V, Meister JJ (2001) Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. J Biomech 34: 1463–1469
Quinn TM, Studer C, Grodzinsky AJ, Meister JJ (2002) Preservation and analysis of nonequilibrium solute concentration distributions within mechanically compressed cartilage explants. J Biochem Biophys Methods 52:83–95
Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH (2001) Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone 29:105–113
Sengers BG, Heywood HK, Lee DA, Oomens CWJ, Bader DL (2005) Nutrient utilization by bovine articular chondrocytes: a combined experimental and theoretical approach. J Biomech Eng 121:758–766
Shao YY, Wang L, Ballock RT (2005) The molecular basis of the Heuter-Volkman Principle of physeal growth. In: Proceedings of the51st Annual Meeting of the Orthopaedic Research Society, Washington DC.,#1054
Shimo T, Gentili C, Iwamoto M, Wu C, Koyama E, Pacifici M (2004) Indian hedgehog land syndecans-3 coregulate chondrocyte proliferation and function during chick limb skeletogenesis. Dev Dyn 229:607–617
Sniekers YH, van Donkelaar CC (2005) Determining diffusion coefficients in inhomogeneous tissues using fluorescence recovery after photobleaching. Biophys J 89:1302–1309
St Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13:2072–2086
Stevens DA, Williams GR (1999) Hormone regulation of chondrocyte differentiation and endochondral bone formation. Mol Cell Endocrinol 151:195–204
Stokes IA, Aronsson DD, Cortright V, Beck S (2005) Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and distraction.In: Proceedings of the 51st Annual Meeting of the Orthopaedic Research Society, Washington DC.,#1047
Tanaka N, Ohno S, Honda K, Tanimoto K, Doi T, Ohno-Nakahara M, Tafolla E, Kapila S, Tanne K (2005) Cyclic mechanical strain regulates the PTHrP expression in cultured chondrocytes via activation of the Ca2+ channel. J Dent Res 84:64–68
Tanck E, Blankevoort L, Haaijman A, Burger EH, Huiskes R (2000) Influence of muscular activity on local mineralization patterns in metatarsals of the embryonic mouse. J Orthop Res 18:613–619
Tanck E, Van Donkelaar CC, Jepsen KJ, Goldstein SA, Weinans H, Burger EH, Huiskes R (2004) The mechanical consequences of mineralization in embryonic bone. Bone 35:186–190
Torzilli PA, Arduino JM, Gregory JD, Bansal M (1997) Effect of proteoglycan removal on solute mobility in articular cartilage. J Biomech 30:895–902
Volk SW, Leboy PS (1999) Regulating the regulators of chondrocyte hypertrophy. J Bone Miner Res 14:483–486
Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ (1996) Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273:613–622
Wu Q, Zhang Y, Chen Q (2001) Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem 276:35290–35296
Zhao Q, Brauer PR, Xiao L, McGuire MH, Yee JA (2002) Expression of parathyroid hormone-related peptide (PthrP) and its receptor (PTH1R) during the histogenesis of cartilage and bone in the chicken mandibular process. J Anat 201:137–151
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Donkelaar, C.C., Huiskes, R. The PTHrP–Ihh Feedback Loop in the Embryonic Growth Plate Allows PTHrP to Control Hypertrophy and Ihh to Regulate Proliferation. Biomech Model Mechanobiol 6, 55–62 (2007). https://doi.org/10.1007/s10237-006-0035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-006-0035-0