Abstract
Background
Rats that recovered from mild proximal tubule (PT) injury without renal dysfunction by subtoxic insult, developed partial resistance to subsequent nephrotoxic insult. This partial resistance was associated with reduced renal dysfunction and accelerated PT cell proliferation compared with vehicle treatment as the first insult. Here we assessed the role and potential mechanisms of accelerated PT proliferation in this acquired resistance model.
Methods
Rats at 14 days after recovering from prior mild renal damage induced by 0.2 mg/kg uranyl acetate (UA) (subtoxic dose) were rechallenged with 4 mg/kg UA (nephrotoxic dose) to establish the acquired resistance model. Cell cycle was inhibited by colchicine to examine the contribution of accelerated PT cell proliferation evaluated by in vivo bromodeoxyuridine (BrdU) labeling on acquired resistance to subsequent nephrotoxic insult. Hepatocyte growth factor (HGF)/c-Met axis and other related factors of cell cycle were analyzed.
Results
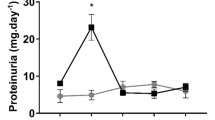
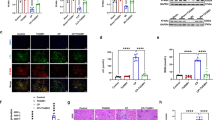
The acquired resistance to rechallenge injury with nephrotoxic dose of UA in rats recovered from mild renal injury was associated with an earlier increase in BrdU-positive PT cells, accelerated upregulation of HGF mRNA, c-Met mRNA/protein, cyclin D1, phospho-Rb and an earlier phenotypic change of PT cells. Colchicine inhibited PT cell proliferation, reduced the upregulated cyclin D1 and phospho-Rb in the kidney, completely abolishing acquired resistance.
Conclusions
Cell cycle progression with upregulated renal HGF/c-Met axis may contribute to the accelerated recovery from acute renal failure in rats that recovered from prior mild renal damage, followed by nephrotoxic insult, resulting in partial acquired resistance.





Similar content being viewed by others
References
Honda N, Hishida A, Ikuma K, Yonemura K. Acquired resistance to acute renal failure. Kidney Int. 1987;31:1233–8.
Furuya R, Kumagai H, Hishida A. Acquired resistance to rechallenge injury with uranyl acetate in LLC-PK1 cells. J Lab Clin Med. 1997;129:347–55.
Zager RA, Burkhart KM, Johnson AC, Sacks BM. Increased proximal tubular cholesterol content: implications for cell injury and “acquired cytoresistance”. Kidney Int. 1999;56:1788–97.
Zager RA. Plasma membrane cholesterol: a critical determinant of cellular energetics and tubular resistance to attack. Kidney Int. 2000;58:193–205.
Kawaida K, Matsumoto K, Shimazu H, Nakamura K. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA. 1994;91:4357–61.
Wang Z, Chen JK, Wang SW, Moeckel G, Harris RC. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol. 2003;14:3147–54.
Villegas G, Lange-Sperandio B, Tufro A. Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int. 2005;67:449–57.
Sharples EJ. Acute kidney injury: stimulation of repair. Curr Opin Crit Care. 2007;13:652–5.
Mizuno S, Fujita K, Furuya R, Hishid A, Ito H, Tashim Y, et al. Association of HSP73 with the acquired resistance to uranyl acetate-induced acute renal failure. Toxicology. 1997;117:183–91.
Sano K, Fujigaki Y, Miyaji T, Ikegaya N, Ohishi K, Yonemura K, et al. Role of apoptosis in uranyl acetate-induced acute renal failure and acquired resistance to uranyl acetate. Kidney Int. 2000;57:1560–70.
Ikuma K, Honda N, Hishida A, Nagase M. Loss of glomerular responses to vasoconstrictor agents in rabbits recovering from ARF. Kidney Int. 1986;30:836–41.
Sun Y, Fujigaki Y, Sakakima M, Hishida A. Acquired resistance to rechallenge injury in rats recovered from subclinical renal damage with uranyl acetate—importance of proliferative activity of tubular cells. Toxicol Appl Pharmacol. 2010;243:104–10.
Mehendale HM, Thakore KN, Rao CV. Autoprotection: stimulated tissue repair permits recovery from injury. J Biochem Toxicol. 1994;9:131–9.
Vaidya VS, Shankar K, Lock EA, Bucci TJ, Mehendale HM. Role of tissue repair in survival from s-(1, 2-dichlorovinyl)-l-cysteine-induced acute renal tubular necrosis in the mouse. Toxicol Sci. 2003;74:215–27.
Korrapati MC, Chilakapati J, Lock EA, Latendresse JR, Warbritton A, Mehendale HM. Preplaced cell division: a critical mechanism of autoprotection against S-1, 2-dichlorovinyl-l-cysteine-induced acute renal failure and death in mice. Am J Physiol Renal Physiol. 2006;291:F439–55.
Fujigaki Y, Sakakima M, Sun Y, Fujikura T, Tsuji T, Yasuda H, et al. Cell division and phenotypic regression of proximal tubular cells in response to uranyl acetate insult in rats. Nephrol Dial Transplant. 2009;24:2686–92.
Matsumoto K, Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59:2023–38.
Liu Y, Tolbert EM, Lin L, Thursby MA, Sun AM, Nakamura T, et al. Up-regulation of hepatocyte growth factor receptor: an amplification and targeting mechanism for hepatocyte growth factor action in acute renal failure. Kidney Int. 1999;55:442–53.
Rao VC, Mehendale HM. Effect of antimitotic agent colchicine on carbon tetrachloride toxicity. Arch Toxicol. 1993;67:392–400.
Dalu A, Rao PS, Mehendale HM. Colchicine antimitosis abolishes resiliency of postnatally developing rats to chlordecone-amplified carbon tetrachloride hepatotoxicity and lethality. Environ Health Perspect. 1998;106:597–606.
Luo J, Tsuji T, Yasuda H, Sun Y, Fujigaki Y, Hishida A. The molecular mechanisms of the attenuation of cisplatin-induced acute renal failure by N-acetylcysteine in rats. Nephrol Dial Transplant. 2008;23:2198–205.
Qiu J, Liu Z, Da L, Li Y, Xuan H, Lin Q, et al. Overexpression of the gene for transmembrane 4 superfamily member 4 accelerates liver damage in rats treated with CCl4. J Hepatol. 2007;46:266–75.
Inoue H, Yokoyama F, Kita Y, Yoshiji H, Tsujimoto T, Deguchi A, et al. Relationship between the proliferative capability of hepatocytes and the intrahepatic expression of hepatocyte growth factor and c-Met in the course of cirrhosis development in rats. Int J Mol Med. 2006;17:857–64.
Del Signore A, De Sanctis V, Di Mauro E, Negri R, Perrone-Capano C, Paggi P. Gene expression pathways induced by axotomy and decentralization of rat superior cervical ganglion neurons. Eur J Neurosci. 2006;23:65–74.
Fujigaki Y, Goto T, Sakakima M, Fukasawa H, Miyaji T, Yamamoto T, et al. Kinetics and characterization of initially regenerating proximal tubules in S3 segment in response to various degrees of acute tubular injury. Nephrol Dial Transplant. 2006;21:41–50.
Li C, Yang CW, Ahn HJ, Kim WY, Park CW, Park JH, et al. Colchicine suppresses osteopontin expression and inflammatory cell infiltration in chronic cyclosporine nephrotoxicity. Nephron. 2002;92:422–30.
Li JJ, Lee SH, Kim DK, Jin R, Jung DS, Kwak SJ, et al. Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297:F200–9.
Terada Y, Inoshita S, Nakashima O, Kuwahara M, Sasaki S, Marumo F. Regulation of cyclin D1 expression and cell cycle progression by mitogen-activated protein kinase cascade. Kidney Int. 1999;56:1258–61.
Seville LL, Shah N, Westwell AD, Chan WC. Modulation of pRB/E2F functions in the regulation of cell cycle and in cancer. Curr Cancer Drug Targets. 2005;5:159–70.
Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol. 2001;280:F562–73.
Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-fos, and clusterin in the post-ischemic kidney. Evidence for a heterogeneous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–88.
Villanueva S, Céspedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol. 2006;290:R861–70.
Liu KD, Brakeman PR. Renal repair and recovery. Crit Care Med. 2008;36:S187–92.
Imai E, Isaka Y. Targeting growth factors to the kidney: myth or reality? Curr Opin Nephrol Hypertens. 2002;11:49–57.
Rabkin R, Fervenza F, Tsao T, Sibley R, Friedlaender M, Hsu F, et al. Hepatocyte growth factor receptor in acute tubular necrosis. J Am Soc Nephrol. 2001;12:531–40.
Tajima H, Higuchi O, Mizuno K, Nakamura T. Tissue distribution of hepatocyte growth factor receptor and its exclusive downregulation in a regenerating organ after injury. J Biochem. 1992;111:401–6.
Leshem Y, Halevy O. Phosphorylation of pRb is required for HGF-induced muscle cell proliferation and is p27kip1-dependent. J Cell Physiol. 2002;191:173–82.
Acknowledgments
This study was presented in part at the 40th annual meeting of the American Society of Nephrology, San Francisco, CA, 2007. This work was supported by a Grant-In-Aid for scientific research (C; No. 19590945) and (C; No. 22590884) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Conflict of interest
There is no declared conflict of interest in this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sun, Y., Fujigaki, Y., Sakakima, M. et al. Acquired resistance to rechallenge injury in rats that recovered from mild renal damage induced by uranyl acetate: accelerated proliferation and hepatocyte growth factor/c-Met axis. Clin Exp Nephrol 15, 666–675 (2011). https://doi.org/10.1007/s10157-011-0453-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-011-0453-x