Abstract
The larval development and survival in the two subantarctic lithodid crabs Lithodes santolla (Jaquinot) and Paralomis granulosa (Molina) from the Argentine Beagle Channel were studied in laboratory cultures. In L. santolla, larval development lasted about 70 days, passing through three zoeal stages and the megalopa stage, with a duration of approximately 4, 7, 11 and 48 days, respectively. The larval development in P. granulosa is more abbreviated, comprising only two zoeal stages and the megalopa stage, with 6, 11 and 43 days' duration, respectively. In both species, we tested for effects of presence versus absence of food (Artemia nauplii) on larval development duration and survival rate. In P. granulosa, we also studied effects of different rearing conditions, such as individual versus mass cultures, as well as aerated versus unaerated cultures. No differences in larval development duration and survival were observed between animals subjected to those different rearing conditions. The lack of response to the presence or absence of potential food confirms, in both species, a complete lecithotrophic mode of larval development. Since lithodid crabs are of high economic importance in the artisanal fishery in the southernmost parts of South America, the knowledge of optimal rearing conditions for lithodid larvae is essential for future attempts at repopulating the collapsing natural stocks off Tierra del Fuego.
Similar content being viewed by others
Introduction
Lithodid crabs are of great importance to the fisheries in high latitudes, both in the northern and southern hemisphere (Dawson 1989, and references therein). In the southernmost parts of Chile and Argentina, they constitute an important part of the local artisanal trap fishery (Campodonico 1971; Lovrich 1997a, 1997b). Lithodes santolla was the first lithodid target species in the Beagle Channel, but recently it has been almost entirely replaced by the smaller lithodid Paralomis granulosa, after the natural stocks of L. santolla had collapsed during the period from 1985 to 1996. The fishery for L. santolla was closed in 1994, and P. granulosa became the new target species. Since 1990, landings of the latter have been variable, averaging around 300 tonnes per year. This level of captures does not seem to affect population parameters of P. granulosa (Lovrich 1997a, 1997b; Lovrich et al. 1998, 1999). However, the slow growth rate of this species suggests that overexploitation of the adult stock may lead to a similar fishery history as previously recorded in L. santolla (Lovrich 1997a, 1997b; Lovrich et al. 1998). Natural recuperation of lithodid stocks is slow, since both species have low growth rates and long generation times of about 6 and 12 years in L. santolla and P. granulosa, respectively. As a consequence of low temperatures, their embryonic development lasts an extremely long time, up to 9–10 and 18–22 months, respectively (Vinuesa 1984; Lovrich and Vinuesa 1993). Furthermore, recent studies of the chemical composition of the larvae (Calcagno et al. 2003; Kattner et al. 2003; Lovrich et al. 2003) suggest that larval development in both species is completely lecithotrophic. This reproductive trait has been interpreted as an adaptation to extreme seasonality in high latitudes characterized by short pulses of planktonic food production. The present work aims to evaluate the effects of various rearing conditions, including availability or lack of food, on larval development duration and survival rates of these two species in the laboratory. This information may help to optimize the rearing of larvae and juveniles for future attempts at repopulating natural lithodid stocks.
Methods
Sampling and maintenance of lithodids
Ovigerous females of the lithodid crabs P. granulosa and L. santolla were caught in April 2000 in the Beagle Channel (54°53.8 S, 68°17.0 W) using commercial fishery traps, and were subsequently kept at 6±0.5°C in aquaria at the local research institute (Centro Austral de Investigaciones Científicas, Ushuaia, Argentina). In May, the crabs were transported by the German research vessel PFS Polarstern to the Marine Biological Station Helgoland (BAH), Germany. During this trip, water was changed three times per week and food (squid meat) was given twice a week. At the BAH, the ovigerous females were maintained individually in flow-through aquaria with a minimum of 20 l water at constant 6±0.5°C, 32‰ salinity, and at an artificial 12:12 h light:dark cycle.
Rearing of larvae
We used sieves with 300 µm mesh size to obtain freshly hatched larvae from the overflow of the aquaria every morning. The sieves were cleaned every evening, thus restricting the variation in larval age to no more than 12 h.
The larvae were randomly selected and transferred individually to 100 ml bowls of seawater. One set of experiments consisted of rearing larvae of P. granulosa and L. santolla in a complete absence of food, while another treatment involved a supply of freshly hatched Artemia nauplii (Argent Chemical Laboratories, USA). Throughout the text, fed larvae are considered as larvae reared under presence of food. Additional experiments with larvae of P. granulosa were performed to compare developmental time and survival in aerated and unaerated cultures, as well as in individual (one larva per 100 ml) and mass cultures (five larvae per 500 ml). Larval cultures were checked daily for deaths and moults. Water was changed every 2 days and, where applicable, potential food (Artemia nauplii) was supplied. The initial number of zoea I in each treatment was 24 for L. santolla and 25 for P. granulosa, both in individual and mass cultures.
Larval development in P. granulosa invariably passed through two zoeal stages and one megalopa, evidenced by the appearance of exuviae and morphological changes (Campodonico and Guzman 1981; McLaughlin et al. 2003). In L. santolla, larval development comprises three zoeal stages and one megalopa stage (Campodonico 1971; McLaughlin et al. 2001). When the megalopa stage was reached, each bowl received a piece of nylon mesh with 200 μm mesh size as an artificial substrate facilitating settlement and metamorphosis. Larvae used in the experiments originated from two females of P. granulosa and from one female of L. santolla.
The statistical comparison of the duration of respective larval stages between different rearing conditions was done with Student's t-test (Sokal and Rohlf 1995). When more than two treatments were involved (e.g. individual vs mass culture, both with and without food), comparisons were made with a one-way analysis of variance (ANOVA). Assumptions of normality and homoscedasticity were tested with Kolmogorov-Smirnov's and Bartlett's tests, respectively.
Results
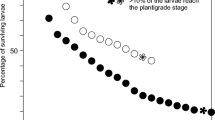
In L. santolla, larval development from hatching to metamorphosis lasted about 70 days, passing through three zoeal stages and a megalopa stage with durations of approximately 4, 7, 11 and 48 days, respectively (Table 1). Larvae of P. granulosa passed through two zoeal stages and a megalopa stage; its complete larval cycle lasted for about 60 days, taking approximately 6 days in zoea I, 11 days in zoea II, and about 43 days in megalopa stage (Table 1). Larvae of both L. santolla and P. granulosa did not feed on the potential food source (Artemia nauplii). In no case were significant differences in the duration of larval development observed between fed and unfed cultures (Student's t-tests, P>0.05; ANOVA P>0.05).
The survival of the larvae of L. santolla was in general slightly higher for unfed larvae than for fed ones, but no statistically significant differences were observed (Table 2). In P. granulosa, likewise, survival was higher in unfed than fed larvae, and it was higher in aerated cultures than in those without air supply. Yet these differences in survival under different conditions of food supply and aeration were not statistically significant either (Table 3).
Discussion
Our results on larval survival and development duration confirm that the two lithodid species P. granulosa and L. santolla follow a completely lecithotrophic and abbreviated larval development. Larvae of both species did not feed on Artemia nauplii, implying complete endotrophic larval development without facultative feeding. In addition, mandibles are poorly developed in the zoeal stages, which should make active feeding impossible, and reach first facultative functionality in the megalopa stage (McLaughlin et al. 2003). This corresponds with recent data on ontogenetic changes in larval biomass and chemical composition, where no influence of the presence or absence of food on the elemental composition (C, N) of king crab larvae was observed (Calcagno et al. 2003; Lovrich et al. 2003). Likewise, the lipid content was not influenced by the presence or absence of food (Kattner et al. 2003). In P. granulosa, however, studies of changes in the elemental composition during larval development (Calcagno et al. 2003) were not fully conclusive, not excluding the possibility of facultative feeding in the final period of the megalopa stage prior to metamorphosis. In the present study on larval development duration and survival rates, no significant differences were observed between fed and unfed larvae of both lithodid species, further supporting the view of an entirely lecithotrophic larval development. This food-independent, i.e. endotrophic, mode of larval development may represent an adaptation to a combination of low average water temperatures (enforcing long larval development duration) and short seasonal periods with sufficient planktonic food availability in subantarctic regions.
In a previous study, Comoglio and Vinuesa (1991) observed effects of different feeding conditions on the larval development of both P. granulosa and L. santolla, with higher initial survival rates in fed cultures. However, larval development was not completed in their experiments, so that no data on the megalopa stage were provided. Another major problem in this study is an apparent mislabelling of their Fig. 1, in which P. granulosa passes through three and L. santolla through two zoeal stages. Besides a confusion between the two species, the data presented in this paper do not correspond with the text. Since larval development in their cultures did not proceed to metamorphosis, the health of the larval material used and/or the quality of the rearing conditions chosen by Comoglio and Vinuesa (1991) remains questionable.
The presence and absence of an additional air supply, and rearing in individual versus mass cultures, had no significant effects on either developmental time or survival of the larvae of both species. Hence, our observations suggest that both L. santolla and P. granulosa are relatively insensitive to culture techniques, which should facilitate future experimental research as well as commercial cultivation or rearing attempts for restocking programmes.
The information provided here may be useful as a basis for future cultivation of subantarctic king crab larvae. In particular, the non-feeding mode of development implies great technical advantages, reducing the time, risks and costs of cultivation on a larger scale. This may help to bypass the high natural mortality occurring during the period of larval development in the plankton. Applied on a pilot scale, production of the first juvenile stage could thus serve for future attempts at re-populating lithodid crabs in the southernmost regions of South America.
References
Calcagno JA, Thatje S, Anger K, Lovrich GA, Kaffenberger A (2003) Changes in biomass and chemical composition during lecithotrophic larval development of the Southern stone crab, Paralomis granulosa (Jacquinot). Mar Ecol Prog Ser 257:189–196
Campodonico I (1971) Desarrollo larval de la centolla Lithodes antarctica Jacquinot en condiciones de laboratorio (Crustacea Decapoda, Anomura: Lithodidae). An Inst Pat Ser Cien Nat (Punta Arenas, Chile) 2:181–190
Campodonico I, Guzman L (1981) Larval development of Paralomis granulosa (Jaquinot) under laboratory conditions (Decapoda, Anomura, Lithodidae). Crustaceana 40:278–285
Campodonico I, Guzman L (1981) Larval development of Paralomis granulosa (Jaquinot) under laboratory conditions (Decapoda, Anomura, Lithodidae). Crustaceana 40:278–285Comoglio L, Vinuesa J (1991) Larval culture of southern king crab Lithodes santolla and false king crab Paralomis granulosa under laboratory conditions. In: Lavens P, Sorgeloos P, Jaspers E, Ollevier F (eds) LARVI '91, fish and crustacean larviculture symposium. European Aquaculture Society, Pec Publ 15, Gent, Belgium, pp349–351
Dawson EW (1989) King crabs of the world (Crustacea: Lithodidae) and their fisheries: a comprehensive bibliography. Misc Publ 101. New Zealand Oceanogr Inst, Div Water Sci, Wellington
Kattner G, Graeve M, Calcagno J, Lovrich G, Thatje S, Anger K (2003) Lipid, fatty acid and protein utilisation during lecithotrophic larval development of Lithodes santolla (Molina) and Paralomis granulosa (Jacquinot). J Exp Mar Biol Ecol 292:61–74
Lovrich GA (1997a) La pesquería mixta de las centollas Lithodes santolla y Paralomis granulosa (Anomura: Lithodidae) en Tierra del Fuego, Argentina. Invest Mar (Valparaíso) 25:41–57
Lovrich GA (1997b) Estado de la pesquería mixta de centolla Lithodes santolla y centollón Paralomis granulosa en el Canal Beagle, Argentina. Informe 1995–1996. Contribución Científica del CADIC No. 25
Lovrich GA, Vinuesa JH (1993) Reproductive biology of the false southern king crab (Paralomis granulosa, Lithodidae) in the Beagle Channel, Argentina. Fish Bull 91:664–675
Lovrich GA, Romero MC, Orozco E (1998) Estado de la pesqueria mixta de centollón Paralomis granulosa y de la centolla Lithodes santolla en el Canal Beagle, Argentina. Informe 1997 Contribución Científica del CADIC No. 31
Lovrich GA, Tapella F, Romero MC (1999) Estado de la pesqueria mixta de centollón Paralomis granulosa y de la centolla Lithodes santolla en el Canal Beagle, Argentina. Informe 1998 Contribución Científica del CADIC No. 33
Lovrich GA, Thatje S, Calcagno JA, Anger K, Kaffenberger A (2003) Changes in biomass and chemical composition during lecithotrophic larval development of the Southern king crab, Lithodes santolla (Molina). J Exp Mar Biol Ecol 288:65–79
McLaughlin PA, Anger K, Kaffenberger A, Lovrich GA (2001) Postlarval development in Lithodes santolla (Molina) (Decapoda: Anomura: Paguroidea: Lithodidae), with notes on zoeal variations. Invertebr Reprod Dev 40:53–67
McLaughlin PA, Anger K, Kaffenberger A, Lovrich GA (2003) Larval and early juvenile development in Paralomis granulosa (Jacquinot) (Decapoda: Anomura: Paguroidea: Lithodidae), with emphasis on abdominal changes in megalopal and crab stages. J Nat Hist 37:1433–1452
Sokal RR, Rohlf FJ (1995) Biometry, the principles and practice of statistics in biological research. Freeman, New York
Vinuesa JH (1984) Sistema reproductor, ciclo y madurez gonadal de la centolla (Lithodes antarcticus) del Canal Beagle. Contr. No. 441, INIDEP, Mar del Plata, Argentina, pp75–95
Acknowledgements
This project was funded by the International Bureau of the German Ministry of Research (BMBF, project No. ARG 99/002), the Argentine Secretaría Nacional para la Tecnología, Ciencia e Inovación Productiva (SETCIP) and the Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, Germany. The first author is grateful to the German Academic Exchange Service (DAAD) for financing his scientific stay at the Biologische Anstalt Helgoland, Germany, in 2000.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.-D. Franke
Rights and permissions
About this article
Cite this article
Calcagno, J.A., Anger, K., Lovrich, G.A. et al. Larval development of the subantarctic king crabs Lithodes santolla and Paralomis granulosa reared in the laboratory. Helgol Mar Res 58, 11–14 (2004). https://doi.org/10.1007/s10152-003-0157-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10152-003-0157-z