Abstract
Purpose
We used an evidence-based approach to determine whether the promotions and claims of superiority of biologic mesh over synthetic mesh use in ventral hernia repairs (VHRs) under contaminated conditions were sound and valid.
Methods
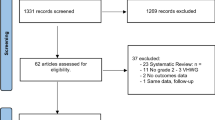
We searched the Medline database to specifically identify review articles relating to biologic mesh and VHR and critically reviewed these studies using an evidence-based approach.
Results
For the past 45 years, four clinical reviews and one systematic review have included biologic meshes as part of a larger discussion on available prosthetics for VHR. All reviews supported biologic mesh use, especially in the setting of contaminated fields. Yet, the primary literature included in these reviews and served as the basis for these conclusions consisted entirely of case series and case reports, which have the lowest level of evidence in determining scientific validity. Furthermore, the FDA has neither cleared nor approved this particular use.
Conclusions
The cumulative data regarding biologic mesh use in VHRs under contaminated conditions does not support the claim that it is better than synthetic mesh used under the same conditions. The highly promoted and at least moderately utilized practice of placing biologic mesh in contamination is being done outside of the original intended use, and a re-evaluation of or possible moratorium on biologic mesh use in hernia surgery is seriously warranted. Alternatively, an industry-sponsored national registry of patients in whom ventral hernia repairs involved biologic mesh would substantively add to our understanding regarding how these intriguing biomaterials are being used and their overall clinical efficacy.
Similar content being viewed by others
References
Shankaran V, Weber DJ, Reed RL II, Luchette FA (2011) A review of available prosthetics for ventral hernia repair. Ann Surg 253(1):16–26. doi:10.1097/SLA.0b013e3181f9b6e6
Gray SH, Hawn MT, Itani KM (2008) Surgical progress in inguinal and ventral incisional hernia repair. Surg Clin N Am 88(1):17–26, vii. doi:10.1016/j.suc.2007.11.007
Basoglu M, Yildirgan MI, Yilmaz I, Balik A, Celebi F, Atamanalp SS, Polat KY, Oren D (2004) Late complications of incisional hernias following prosthetic mesh repair. Acta Chir Belg 104(4):425–428
Morris-Stiff GJ, Hughes LE (1998) The outcomes of nonabsorbable mesh placed within the abdominal cavity: literature review and clinical experience. J Am Coll Surg 186(3):352–367
Hiles M, Record Ritchie RD, Altizer AM (2009) Are biologic grafts effective for hernia repair. Surg Innov 16(1):26–37
Bachman S, Ramshaw B (2008) Prosthetic material in ventral hernia repair: how do I choose? Surg Clin N Am 88(1):101–112, ix. doi:10.1016/j.suc.2007.11.001
Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D (2010) Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 148(3):544–558. doi:10.1016/j.surg.2010.01.008
Hodde J (2006) Extracellular matrix as a bioactive material for soft tissue reconstruction. ANZ J Surg 76(12):1096–1100. doi:10.1111/j.1445-2197.2006.03948.x
Rosen MJ (2010) Biologic mesh for abdominal wall reconstruction: a critical appraisal. Am Surg 76(1):1–6
21 CFR 800-1299
Segan RD (2010) A response to “Major complications associated with xenograft biologic mesh implantation in abdominal wall reconstruction” (Harth KC, Rosen MJ. Surg Innov. 2009;16:324–329) and discussion of the MAUDE (manufacturer and user facility device experience) database, FDA regulation of biologic implants, and evidence-based medicine. Surg Innov 17 (3):273-275; author reply 276. doi:10.1177/1553350610371214
21 CFR 873.3300
21 CFR 812
Rosenburg W, Donald A (1995) Evidence based medicine: an approach to clinical problem-solving. Br Med J 310:1122–1126
Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS (1996) Evidence based medicine: what it is and what it isn’t. BMJ 312(7023):71–72
US Preventive Services Task Force Ratings: Strength of Recommendations and Quality of Evidence (Periodic updates, 2000–2003) Guide to clinical preventive services. 3rd edn. Agency for Healthcare Research and Quality, Rockville
Petrisor BA, Bhandari M (2007) The heirarchy of evidence: levels and grades of recommendation. Indian J Orthoped 41:11–15
Phillips B, Ball C, Sackett D, et al (2011) Oxford Center for Evidence-Based Medicine-Levels of Evidence. Center for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=1025. Accessed March 22 2010
Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, Boelhouwer RU, de Vries BC, Salu MK, Wereldsma JC, Bruijninckx CM, Jeekel J (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343(6):392–398. doi:10.1056/NEJM200008103430603
Penttinen R (2008) Mesh Repair of common abdominal hernias: a review on experimental and clinical studies. Hernia 12:337–344
Ghazi B, Deigni O, Yezhelyev M, Losken A (2011) Current options in the management of complex abdominal wall defects. Ann Plast Surg 66(5):488–492
Kim H, Bruen K, Vargo D (2006) Acellular dermal matrix in the management of high-risk abdominal wall defects. Am J Surg 192(6):705–709. doi:10.1016/j.amjsurg.2006.09.003
Patton JH Jr, Berry S, Kralovich KA (2007) Use of Human acellular dermal matrix in complex and contaminated abdominal wall reconstructions. Am J Surg 193(3):360–363
Diaz JJ Jr, Guy J, Berkes MB, Guillamondegui O, Miller RS (2006) Acellular dermal allograft for ventral hernia repair in the compromised surgical field. Am Surg 72(12):1181–1187 Discussion 1187–1188
Schuster R, Singh J, Safadi BY et al (2006) The use of acellular dermal matrix for contaminated abdominal wall defects: wound status predicts success. Am J Surg 192(5):594–597
Catena F, Ansaloni L, Gazzotti F, Gagliardi S, Di Saverio S, D’Alessandro L, Pinna AD (2007) Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia 11(1):57–60
Ueno T, Pickett LC, de la Fuente SG, Lawson DC, Pappas TN (2004) Clinical application of porcine small intestinal submucosa in the management of infected or potentially contaminated abdominal defects. J Gastrointest Surg 8(1):109–112
Helton WS, Fisichella PM, Berger R, Horgan S, Espat NJ, Abcarian H (2005) Short-term outcomes with small intestinal submucosa for ventral abdominal hernia. Arch Surg 140(6):549–560. doi:10.1001/archsurg.140.6.549. Discussion 560–542
Franklin ME Jr, Gonzalez JJ Jr, Michaelson RP, Glass JL, Chock DA (2002) Preliminary experience with new bioactive prosthetic material for repair of hernias in infected fields. Hernia 6(4):171–174. doi:10.1007/s10029-002-0078-9
Trevino JM, Franklin ME Jr, Berghoff KR, Glass JL, Jaramillo EJ (2006) Preliminary results of a two-layered prosthetic repair for recurrent inguinal and ventral hernias combining open and laparoscopic techniques. Hernia 10(3):253–257. doi:10.1007/s10029-006-0085-3
Diaz JJ Jr, Conquest AM, Ferzoco SJ, Vargo D, Miller P, Wu YC, Donahue R (2009) Multi-institutional experience using human acellular dermal matrix for ventral hernia repair in a compromised surgical field. Arch Surg 144(3):209–215. doi:10.1001/archsurg.2009.12
Alaedeen DI, Lipman J, Medalie D, Rosen MJ (2007) The single-staged approach to the surgical management of abdominal wall hernias in contaminated fields. Hernia 11(1):41–45. doi:10.1007/s10029-006-0164-5
Parker DM, Armstrong PJ, Frizzi JD, North JH Jr (2006) Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg 63(4):255–258. doi:10.1016/j.cursur.2006.05.003
Bellows CF, Albo D, Berger DH, Awad SS (2007) Abdominal wall repair using human acellular dermis. Am J Surg 194(2):192–198. doi:10.1016/j.amjsurg.2006.11.012
Bluebond-Langner R, Keifa ES, Mithani S, Bochicchio GV, Scalea T, Rodriguez ED (2008) Recurrent abdominal laxity following interpositional human acellular dermal matrix. Ann Plast Surg 60(1):76–80. doi:10.1097/SAP.0b013e31804efcbc
Kolker AR, Brown DJ, Redstone JS, Scarpinato VM, Wallack MK (2005) Multilayer reconstruction of abdominal wall defects with acellular dermal allograft (AlloDerm) and component separation. Ann Plast Surg 55(1):36–41 Discussion 41–32
Butler CE, Langstein HN, Kronowitz SJ (2005) Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg 116(5):1263–1275 Discussion 1276–1267
Adedeji OA, Bailey CA, Varma JS (2002) Porcine dermal collagen graft in abdominal-wall reconstruction. Br J Plast Surg 55(1):85–86. doi:10.1054/bjps.2001.3711
Liyanage SH, Purohit GS, Frye JN, Giordano P (2006) Anterior abdominal wall reconstruction with a Permacol implant. J Plast Reconstr Aesthet Surg 59(5):553–555
Tung CS, Zighelboim I, Scott B, Anderson ML (2006) Human acellular dermal matrix for closure of a contaminated gynecologic wound. Gynecol Oncol 103(1):354–356
Gupta A, Zahriya K, Mullens PL, Salmassi S et al (2007) Experience with two different biosynthetic mesh materials, Surgisis and Alloderm. Hernia 10:419–425
Buinewicz B, Rosen B (2004) Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 52(2):188–194. doi:10.1097/01.sap.0000100895.41198.27
Jin J, Rosen MJ, Blatnik J, McGee MF, Williams CP, Marks J, Ponsky J (2007) Use of acellular dermal matrix for complicated ventral hernia repair: does techique affect outcomes? J Am Coll Surg 205:600–654
Candage R, Jones K, Luchette FA, Sinacore JM, Vandevender D, Reed RL 2nd (2008) Use of human acellular dermal matrix for hernia repair: friend or foe? Surgery 144(4):703–711
Brown GL, Richardson JD, Malangoni MA, Tobin GR, Ackerman D, Polk HC Jr (1985) Comparison of prosthetic materials for abdominal wall reconstruction in the presence of contamination and infection. Ann Surg 201(6):705–711
Bleichrodt RP, Simmermacher RK, van der Lei B, Schakenraad JM (1993) Expanded polytetrafluoroethylene patch versus polypropylene mesh for the repair of contaminated defects of the abdominal wall. Surg Gynecol Obstet 176(1):18–24
Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J (2004) Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 240(4):578–583 Discussion 583–575
Leber GE, Garb JL, Alexander AI, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133(4):378–382
van Geffen H, Simmermacher RK, van Vroonhoven T, van der Werken C (2005) Surgical treatment of large contaminated abdominal wall defects. J Am Coll Surg 201:206–212
Szczerba SR, Dumanian GA (2003) Definitive surgical treatment of infected or exposed ventral hernia mesh. Ann Surg 237(3):437–441. doi:10.1097/01.SLA.0000055278.80458.D0
Voyles CR, Richardson JD, Bland KI, Tobin GR, Flint LM, Polk HC Jr (1981) Emergency abdominal wall reconstruction with polypropylene mesh: short-term benefits versus long-term complications. Ann Surg 194(2):219–223
Jones JW, Jurkovich GJ (1989) Polypropylene mesh closure of infected abdominal wounds. Am Surg 55(1):73–76
Paton BL, Novitsky YW, Zerey M, Sing RF, Kercher KW, Heniford BT (2007) Management of infections of polytetrafluoroethylene-based mesh. Surg Infect (Larchmt) 8(3):337–341. doi:10.1089/sur.2006.053
Howes N, Chagla L, Thorpe M, McCulloch P (1997) Surgical practice is evidence based. Br J Surg 84:1220–1223
Wente M, Seiler C, Uhl W, Buchler M (2003) Perspectives of evidence-based surgery. Dig Surg 20:263–269
Harth K, Rosen J (2011) Major complications associated with xenograft biologic mesh implantation in abdominal wall reconstruction. Surg Innov 16(4):324–329
Hirsch EF (2004) Repair of an abdominal wall defect after a salvage laparotomy for sepsis. J Am Coll Surg 198(2):324–328. doi:10.1016/j.jamcollsurg.2003.09.016
Armellino MF, De Stefano G, Scardi F et al (2006) Use of Permacol in complicated incisional hernia (in Italian). Chir Ital 58(5):627–630
Acknowledgments
We would like to thank Pamela Derish in the Department of Surgery at UCSF for her editing expertise and technical assistance. This manuscript was supported by the NIH 5 T32 Grant DK07573-23; “Research Training in Gastrointestinal Surgery”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Primus, F.E., Harris, H.W. A critical review of biologic mesh use in ventral hernia repairs under contaminated conditions. Hernia 17, 21–30 (2013). https://doi.org/10.1007/s10029-012-1037-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-012-1037-8