Abstract
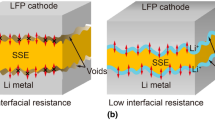
Organic ionic plastic crystal (OIPC) electrolytes are among the key enabling materials for solid-state and higher than ambient temperature lithium batteries. This work overviews some of the parameter studies on the Li|OIPC interface using lithium symmetrical cells as well as the optimisation and performance of Li|OIPC|LiFePO4 cells. The effects of temperature and electrolyte thickness on the cycle performance of the lithium symmetrical cell, particularly with respect to the interfacial and bulk resistances, are demonstrated. Whilst temperature change substantially alters both the interfacial and bulk resistance, changing the electrolyte thickness predominantly changes the bulk resistance only. In addition, an upper limit of the current density is demonstrated, above which irreversible processes related to electrolyte decomposition take place. Here, we demonstrate an excellent discharge capacity attained on LiFePO4|10 mol% LiNTf2-doped [C2mpyr][NTf2]|Li cell, reaching 126 mAh g-1 at 50 °C (when the electrolyte is in its solid form) and 153 mAh g-1 at 80 °C (when the electrolyte is in its liquid form). Most remarkably, at high temperature operation, the capacity retention at long cycles and high current is excellent with only a slight (3%) drop in discharge capacity upon increasing the current from 0.2 C to 0.5 C. These results highlight the real prospects for developing a lithium battery with high temperature performance that easily surpasses that achievable with even the best contemporary lithium-ion technology.






Similar content being viewed by others
References
Seki S, Ohno Y, Kobayashi Y, Miyashiro H, Usami A, Mita Y, Tokuda H, Watanabe M, Hayamizu K, Tsuzuki S, Hattori M, Terada N (2007) J Electrochem Soc 154:A173–A177
Zhou Z-B, Matsumoto H (2007) Electrochem Commun 9:1017–1022
Abouimrane A, Whitfield PS, Niketic S, Davidson IJ (2007) J Power Sources 174:883–888
Abouimrane A, Abu-Lebdeh Y, Alarco P-J, Armand M (2004) J Electrochem Soc 151:A1028–A1031
Alarco P-J, Abu-Lebdeh Y, Ravet N, Armand M (2004) Solid State Ionics 172:53–56
Abu-Lebdeh Y, Abouimrane A, Alarco P-J, Armand M (2006) J Power Sources 154:255–261
MacFarlane DR, Meakin P, Amini N, Forsyth M (2001) J Phys Condens Matter 13:8257–8267
MacFarlane DR, Forsyth M (2001) Adv Mater 13:957–966
Forsyth M, Huang JH, MacFarlane DR (2000) J Mater Chem 10:2259–2265
MacFarlane DR, Huang J, Forsyth M (1999) Nature 402:792–794
Annat G, Adebahr J, McKinnon IR, MacFarlane DR, Forsyth M (2007) Solid State Ionics 178:1065–1071
Howlett PC, Shekibi Y, MacFarlane DR, Forsyth M (2009) Adv Eng Mater 11:1044–1048
Howlett PC, Sunarso J, Shekibi Y, Wasser E, Jin L, Kar M, MacFarlane D, Forsyth M (2011) Solid State Ionics (submitted for publication)
Armel V, Velayutham D, Sun J, Howlett PC, Forsyth M, MacFarlane DR, Pringle JM (2011) J Mater Chem 21:7640–7650
MacFarlane D, Meakin P, Sun J, Amini N, Forsyth M (1999) J Phys Chem B 103:4164–4170
Bhatt AI, Best AS, Huang J, Hollenkamp AF (2010) J Electrochem Soc 157:A66–A74
Acknowledgements
The authors gratefully acknowledge the financial support from the Australian Research Council (ARC) through the ARC Centre of Excellence for Electromaterials Science and ARC Laureate Fellowship for Maria Forsyth. Jaka Sunarso acknowledges Alfred Deakin Postdoctoral Research Fellowship from Deakin University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sunarso, J., Shekibi, Y., Efthimiadis, J. et al. Optimising organic ionic plastic crystal electrolyte for all solid-state and higher than ambient temperature lithium batteries. J Solid State Electrochem 16, 1841–1848 (2012). https://doi.org/10.1007/s10008-011-1566-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-011-1566-6