Abstract
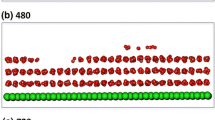
The adsorption of molecular hydrogen on few-layer graphene (FLG) structures is studied using molecular dynamics simulations. The interaction between graphene and hydrogen molecules is described by the Lennard-Jones potential. The effects of pressure, temperature, number of layers in a FLG, and FLG interlayer spacing are evaluated in terms of molecular trajectories, binding energy, binding force, and gravimetric hydrogen storage capacity (HSC). The simulation results show that the effects of temperature and pressure can offset each other to improve HSC. An insufficient interlayer spacing (0.35 nm) largely limits the HSC of FLG because hydrogen adsorbed at the edges of the graphene prevents more hydrogen from entering the structure. A low temperature (77 K), a high pressure, a large number of layers in a FLG, and a large FLG interlayer spacing maximize the HSC.







Similar content being viewed by others
References
Hwang JJ (2012) Review on development and demonstration of hydrogen fuel cell scooters. Renew Sustain Energy Rev 16(6):3803–3815
Lee C, Wei X, Kysar W, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321:385–388
Castro Neto AH, Guinea F, Peres NMR, Novoselov KS, Geim AK (2009) The electronic properties of graphene. Rev Mod Phys 81:109–162
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282–286
Schlapbach L, Zuttel A (2001) Hydrogen-storage materials for mobile applications. Nature 414:353–358
Wu CD, Fang TH, Lin JF (2012) Atomic-scale simulations of material behaviors and tribology properties for FCC and BCC metal films. Mater Lett 80:59–62
Sung PH, Wu CD, Fang TH, Weng CI (2012) Size effect on shape recovery and induced strain of NiTi nanowires. Appl Surf Sci 258(18):7064–7069
Sung PH, Wu CD, Fang TH (2012) Effects of temperature, loading rate, and nanowire length on torsional deformation and mechanical properties of aluminum nanowires investigated using molecular dynamics simulation. J Phys D: Appl Phys 45:215303-1-8 doi:10.1088/0022-3727/45/21/215303
Hsu QC, Lin YT, Chou DC, Wu CD (2012) Study on nanoimprint formability considered anti-adhesion layer for the (CH2)n polymer material by molecular dynamics simulation. Curr Nanosci 8(3):424–431
Wu CD, Fang TH, Wu TT (2012) Effects of humidity and temperature on laser-assisted dip-pen nanolithography array studied using molecular dynamics simulations. J Colloid Interface Sci 372(1):170–175
Wu CD, Fang TH, Lin JF (2012) Effects of tip gap, deposition temperature, holding time, and pull-off velocity on dip-pen lithography using molecular dynamics simulation. J Appl Phys 111 (10):103521-1-8 doi:10.1063/1.4720576
Herrero CP, Ramirez R (2010) Diffusion of hydrogen in graphite: a molecular dynamics simulation. J Phys D: Appl Phys 43:255402-1-7 doi:10.1088/0022-3727/43/25/255402
Ferro Y, Marinelli F, Allouche A (2002) Density functional theory investigation of H adsorption and H2 recombination on the basal plane and in the bulk of graphite: connection between slab and cluster model. J Chem Phys 116:8124–8131
Dino WA, Miura Y, Nakanishi H, Kasai H, Sugimoto T (2003) Stable hydrogen configurations between graphite layers. J Phys Soc Jpn 72:1867–1870
Lamari FD, Levesque D (2011) Hydrogen adsorption on functionalized graphene. Carbon 49:5196–5200
Tozzini V, Pellegrini V (2011) Reversible hydrogen storage by controlled buckling of graphene layers. J Phys Chem C 115:25523–25528
Sofo JO, Chaudhari AS, Barber GD (2007) Graphane: a two-dimensional hydrocarbon. Phys Rev B 75:153401-1-4 doi:10.1103/PhysRevB.75.153401
Haile JM (1992) Molecular dynamics simulation: elementary methods. Wiley, New York
Simon JM, Haas OE, Kjelstrup S (2010) Adsorption and desorption of H2 on graphite by molecular dynamics simulations. J Phys Chem C 114:10212–10220
Henwood D, David Carey J (2007) Ab initio investigation of molecular hydrogen physisorption on graphene and carbon nanotubes. Phys Rev B 75:245413-1-10 doi:10.1103/PhysRevB.75.245413
Dimitrakakis GK, Tylianakis E, Froudakis GE (2008) Pillared graphene: a new 3-D network nanostructure for enhanced hydrogen storage. Nano Lett 8:3166–3170
Reina A, Jia X, Ho J, Nezich D, Son H, Bulovic V, Dresselhaus MS, Kong J (2009) Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett 9(1):30–35
Zhao H, Min K, Aluru NR (2009) Size and chirality dependent elastic properties of graphene nanoribbons under uniaxial tension. Nano Lett 9:3012–3015
Poirier E, Chahine R, Benard P, Cossement D, Lafi L, Melancon E, Bose TK, Desilets S (2004) Storage of hydrogen on single-walled carbon nanotubes and other carbon structures. Appl Phys A 78:961–967
Wang L, Stuckert NR, Yang RT (2011) Unique hydrogen adsorption properties of graphene. AICHE J 57:2902–2908
Tibbetts GG, Meisner GP, Olk CH (2001) Hydrogen storage capacity of carbon nanotubes, filaments, and vapor-grown fibers. Carbon 39:2291–2301
Zhu HW, Ci LJ, Chen A, Mao ZQ, Xu CL, Xiao X, Wei BQ, Liang J, Wu DH (2000) Hydrogen uptake in multi-walled carbon nanotubes at room temperature. In: Mao ZQ, Veziroglu TN (eds) Hydrogen energy progress XIII, Proceedings of the 13th world hydrogen energy conference. International association for hydrogen energy, Beijing, pp 560–564
Wu HB, Chen P, Lin J, Tan KL (2000) Hydrogen uptake by carbon nanotubes. Int J Hydrogen Energy 25:261–265
Browning DJ, Gerrard ML, Laakeman JB, Mellor IM, Mortimer RJ, Turpin MC (2000) Investigation of the hydrogen storage capacities of carbon nanofibres prepared from an ethylene precursor. In: Mao ZQ, Veziroglu TN (eds) Hydrogen energy progress XIII, Proceedings of the 13th world hydrogen energy conference. International association for hydrogen energy, Beijing, pp 554–559
Gupta BK, Awasthi K, Srivastava ON (2000) New Carbon Variants: Graphitic nanofibres and nanotubules as hydrogen storage materials. In: Mao ZQ, Veziroglu TN (eds) Hydrogen energy progress XIII, Proceedings of the 13th world hydrogen energy conference. International association for hydrogen energy, Beijing, pp 487–492
Darkrim F, Levesque D (2000) High adsorptive property of opened carbon nanotubes at 77 K. J Phys Chem B 104:6773–6776
Phillips AB, Shivaram BS (2008) High capacity hydrogen absorption in transition metal-ethylene complexes observed via nanogravimetry. Phys Rev Lett 100:105505-1-4 doi:10.1103/PhysRevLett.100.105505
Kalamse V, Wadnerkar N, Chaudhari A (2013) Multi-functionalized naphthalene complexes for hydrogen storage. Energy 49:469–474
Phillips AB, Shivaram BS, Myneni GR (2012) Hydrogen absorption at room temperature in nanoscale titanium benzene complexes. Int J Hydrogen Energy 37:1546–1550
Kalamse V, Wadnerkar N, Deshmukh A, Chaudhari A (2012) Interaction of molecular hydrogen with Ni doped ethylene and acetylene complex. Int J Hydrogen Energy 37:5114–5121
Kalamse V, Wadnerkar N, Chaudhari A (2010) Hydrogen storage in C2H4V and C2H4V+ organometallic compounds. J Phys Chem C 114:4704–4709
Kalamse V, Wadnerkar N, Deshmukh A, Chaudhari A (2012) C2H2M (M = Ti, Li) complex: a possible hydrogen storage material. Int J Hydrogen Energy 37:3727–3732
Acknowledgments
This work was supported by the National Science Council of Taiwan under grants NSC 100-2628-E-151-003-MY3 and NSC 100-2221-E-151-018-MY3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, CD., Fang, TH., Lo, JY. et al. Molecular dynamics simulations of hydrogen storage capacity of few-layer graphene. J Mol Model 19, 3813–3819 (2013). https://doi.org/10.1007/s00894-013-1918-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1918-5