Abstract
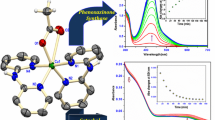
[Cu2+•Cys-Gly-His-Lys] stimulates thermolysin (TLN) activity at low concentration (below 10 μM) and inhibits the enzyme at higher concentration, with binding affinities of 2.0 and 4.9 μM, respectively. The metal-free Cys-Gly-His-Lys peptide also stimulates TLN activity, with an apparent binding affinity of 2.2 μM. Coordination of copper through deprotonated imine nitrogens, the histidyl nitrogen, and the free N-terminal amino group is consistent with the characteristic absorption spectrum of a Cu2+–amino-terminal copper and nickel binding motif (λ max ∼ 525 nm). The lack of thiol coordination is suggested by both the absence of a thiol to Cu2+ charge transfer band and electrochemical studies, since the electrode potential (vs. Ag/AgCl) 0.84 V (ΔE = 92 mV) for the Cu3+/2+ redox couple obtained for [Cu2+•Cys-Gly-His-Lys] was found to be in close agreement with that of a related complex [Cu2+•Lys-Gly-His-Lys]+ (0.84 V, ΔE = 114 mV). The N-terminal cysteine appears to be available as a zinc-anchoring residue and plays a critical functional role since the [Cu2+•Lys-Gly-His-Lys]+ homologue exhibits neither stimulation nor inhibition of TLN. Under oxidizing conditions (ascorbate/O2) the catalyst is shown to mediate the complete irreversible inactivation of TLN at concentrations where enzyme activity would otherwise be stimulated. The observed rate constant for inactivation of TLN activity was determined as k obs = 7.7 × 10−2 min−1, yielding a second-order rate constant of (7.7 ± 0.9) × 104 M−1 min−1. Copper peptide mediated generation of reactive oxygen species that subsequently modify active-site residues is the most likely pathway for inactivation of TLN rather than cleavage of the peptide backbone.







Similar content being viewed by others
References
Endo S (1962) Hakko Kogaku Zasshi 40:346–353
Latt SA, Holmquist B, Vallee BL (1969) Biochem Biophys Res Commun 37:333–339
Feder J, Garrett LR, Wildi BS (1971) Biochemistry 10:4552–4556
Roques BP, Noble F, Dauge V, Fournie-Zaluski M-C, Beaumont A (1993) Pharmacol Rev 45:87–146
Cheng X-M, Ahn K, Haleen SJ (1997) Annu Rep Med Chem 32:61–70
Wyvratt MJ, Patchett AA (1985) Med Res Rev 5:483–531
Roques BP (1993) Biochem Soc Trans 21:678–685
Matthews BW (1988) Acc Chem Res 21:333–340
Holmes MA, Matthews BW (1982) J Mol Biol 160:623–639
Tiraboschi G, Jullian N, Thery V, Antonczak S, Fournie-Zaluski MC, Roques BP (1999) Protein Eng 12:141–149
Bohacek R, De Lombaert S, McMartin C, Priestle J, Gruetter M (1996) J Am Chem Soc 118:8231–8249
Fillion E, Gravel D (1996) Bioorg Med Chem Lett 6:2097–2102
Gokhale NH, Cowan JA (2005) Chem Commun 5916–5918
Gokhale NH, Cowan JA (2006) J Biol Inorg Chem 11:937–947
Cushman DW, Cheung HS, Sabo EF, Ondetti MA (1977) Biochemistry 16:5484–5491
Gaucher JF, Selkti M, Tiraboschi G, Prange T, Roques BP, Tomas A, Fournie-Zaluski MC (1999) Biochemistry 38:12569–12576
Gomez-Monterrey I, Beaumont A, Nemecek P, Roques BP, Fournie-Zaluski M-C (1994) J Med Chem 37:1865–1873
Holmquist B, Vallee BL (1979) Proc Natl Acad Sci USA 76:6216–6220
Peters K, Jahreis G, Kotters E-M (2001) J Enzyme Inhib 16:339–350
Roderick SL, Fournie-Zaluski MC, Roques BP, Matthews BW (1989) Biochemistry 28:1493–1497
Harford C, Sarkar B (1997) Acc Chem Res 30:123–130
McDonald MR, Scheper WM, Lee HD, Margerum DW (1995) Inorg Chem 34:229–237
Tesfai TM, Green BJ, Margerum DW (2004) Inorg Chem 43:6726–6733
Johnson GD, Ahn K (2000) Anal Biochem 286:112–118
Segel I (1993) Enzyme kinetics: behavior and analysis of rapid equilibium and steady-state enzyme systems. Wiley Interscience, New York
Suh J (2003) Acc Chem Res 36:562–570
Acknowledgement
We thank the National Institutes of Health for financial support of this work (GM 63740) to J.A.C.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gokhale, N.H., Bradford, S. & Cowan, J.A. Stimulation and oxidative catalytic inactivation of thermolysin by copper•Cys-Gly-His-Lys. J Biol Inorg Chem 12, 981–987 (2007). https://doi.org/10.1007/s00775-007-0270-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-007-0270-6