Abstract
Purpose
Malignant gliomas are highly infiltrative tumours with a fatal prognosis. F18-fluoroethyl-tyrosine (FET)-positron emission tomography (PET) often reveals a broader extension of these tumours compared with contrast-enhanced magnetic resonance imaging (MRI). Complete resection of the contrast-enhancing lesion is aspired. Fluorescence-guided resection using 5-aminolevulinic acid (5-ALA) improved the extent of resection. In this study, we investigated whether the FET uptake correlates with the extent of resection using 5-ALA-induced fluorescence.
Methods
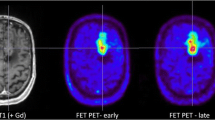
Thirteen patients who underwent preoperative and postoperative MRI, FET-PET and fluorescence-guided neuronavigated resection were included in this study. The areas in which intraoperative fluorescence terminated the resection were marked. After fusion of PET and MRI, the standardized uptake value (SUV) of FET related to normal brain (SUVR) was measured in regions of interest corresponding to resected and remaining tissue, respectively. Receiver-operating characteristic (ROC) curve analysis determined the optimal threshold of the relative SUV anticipating 5-ALA-induced fluorescence.
Results
During resection a vivid fluorescence was present in all patients. Histology revealed glioblastomas in 11 cases, an anaplastic astrocytoma in one case and a low-grade astrocytoma in one case. The median FET SUVR was higher in areas corresponding to the fluorescent tumour compared with the non-fluorescent normal brain (2.321 vs 1.142, p < 0.0001, t-test). A SUVR greater than 1.374 predicted the fluorescence with a sensitivity of 0.87 [95% confidence interval (CI): 0.74–0.94] and a specificity of 0.94 (CI: 0.84–0.99). The area under the ROC curve was 0.9656 (CI: 0.9364–0.9948).
Conclusions
FET uptake predicts the 5-ALA-induced fluorescence in glioma patients. Thus, FET-PET provides useful information for planning glioma resection.






Similar content being viewed by others
References
Beck TJ, Kreth FW, Beyer W, Mehrkens JH, Obermeier A, Stepp H, Stummer W, Baumgartner R (2007) Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg Med 39:386–393
Brambilla M, Secco C, Dominietto M, Matheoud R, Sacchetti G, Inglese E (2005) Performance characteristics obtained for a new 3-dimensional lutetium oxyorthosilicate-based whole-body PET/CT scanner with the National Electrical Manufacturers Association NU 2-2001 standard. J Nucl Med 46:2083–2091
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Burger PC, Heinz ER, Shibata T, Kleihues P (1988) Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg 68:698–704
Ennis SR, Novotny A, Xiang J, Shakui P, Masada T, Stummer W, Smith DE, Keep RF (2003) Transport of 5-aminolevulinic acid between blood and brain. Brain Res 959:226–234
Floeth FW, Sabel M, Stoffels G, Pauleit D, Hamacher K, Steiger HJ, Langen KJ (2008) Prognostic value of 18F-fluoroethyl-L-tyrosine PET and MRI in small nonspecific incidental brain lesions. J Nucl Med 49:730–737
Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H (2008) 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institutuion. Swiss Med Wkly 138:180–185
Henze M, Mohammed A, Schlemmer HP, Herfarth KK, Hoffner S, Haufe S, Mier W, Eisenhut M, Debus J, Haberkorn U (2004) PET and SPECT for detection of tumor progression in irradiated low-grade astrocytoma: a receiver-operating-characteristic analysis. J Nucl Med 45:579–586
Ishihara R, Katayama Y, Watanabe T, Yoshino A, Fukushima T, Sakatani K (2007) Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol Med Chir (Tokyo) 47:53–57 discussion 57
Krengli M, Loi G, Sacchetti G, Manfredda I, Gambaro G, Brambilla M, Carriero A, Inglese E (2007) Delineation of target volume for radiotherapy of high-grade gliomas by 99m Tc-MIBI SPECT and MRI fusion. Strahlenther Onkol 183:689–694
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, Zilles K, Coenen HH, Langen KJ (2005) O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128:678–687
Pichlmeier U, Bink A, Schackert G, Stummer W (2008) Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol 10:1025–1034
Plotkin M, Gneveckow U, Meier-Hauff K, Amthauer H, Feussner A, Denecke T, Gutberlet M, Jordan A, Felix R, Wust P (2006) 18F-FET PET for planning of thermotherapy using magnetic nanoparticles in recurrent glioblastoma. Int J Hyperthermia 22:319–325
Popperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K (2004) Value of O-(2-[18F]fluoroethyl)- L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 31:1464–1470
Shibata Y, Yamamoto T, Takano S, Katayama W, Takeda T, Matsumura A (2009) Direct comparison of thallium-201 and technetium-99m MIBI SPECT of a glioma by receiver operating characteristic analysis. J Clin Neurosci 16:264–269
Stober B, Tanase U, Herz M, Seidl C, Schwaiger M, Senekowitsch-Schmidtke R (2006) Differentiation of tumour and inflammation: characterisation of [methyl-3H]methionine (MET) and O-(2-[18F]fluoroethyl)-L-tyrosine (FET) uptake in human tumour and inflammatory cells. Eur J Nucl Med Mol Imaging 33:932–939
Stockhammer F, Plotkin M, Amthauer H, van Landeghem FK, Woiciechowsky C (2008) Correlation of F-18-fluoro-ethyl-tyrosin uptake with vascular and cell density in non-contrast-enhancing gliomas. J Neurooncol 88:205–210
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, Rohde V, Oppel F, Turowski B, Woiciechowsky C, Franz K, Pietsch T (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62:564–576 discussion 564–576
Stummer W, Stocker S, Novotny A, Heimann A, Sauer O, Kempski O, Plesnila N, Wietzorrek J, Reulen HJ (1998) In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B 45:160–169
Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, Goetz AE, Kiefmann R, Reulen HJ (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42:518–525 discussion 525–516
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Watanabe M, Tanaka R, Takeda N (1992) Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 34:463–469
Weber DC, Zilli T, Buchegger F, Casanova N, Haller G, Rouzaud M, Nouet P, Dipasquale G, Ratib O, Zaidi H, Vees H, Miralbell R (2008) [(18)F]Fluoroethyltyrosine-positron emission tomography-guided radiotherapy for high-grade glioma. Radiat Oncol 3:44
Willems PW, Taphoorn MJ, Burger H, Berkelbach van der Sprenkel JW, Tulleken CA (2006) Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg 104:360–368
Winkler F, Kienast Y, Fuhrmann M, Von Baumgarten L, Burgold S, Mitteregger G, Kretzschmar H, Herms J (2009) Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The authors present a retrospective study on a limited series of patients affected by gliomas in order to assess the concordance of preoperative FET-PET uptake values and intraoperative 5-ALA-induced fluorescence data. Standard MRI sequences are usually used for surgical planning, even though they are only able to partially detect the extent and, even less, the biological features of gliomas. Therefore, every effort to investigate the relationships between preoperative images and intraoperative data is welcome in order to achieve a more effective use of surgery in neuro-oncology. In this series the authors show that preoperative FET-PET is an accurate predictor of 5-ALA fluorescence-based tumour resection. Consequently, it can represent a useful tool to plan and conduct surgery. Nevertheless, we have to remember that both techniques are able to primarily detect the amount of tumoural cellularity and for this reason they are able to show only one aspect of the tumour biology, which is usually heterogeneous in this kind of tumour (consider, for example, the variable degree of necrosis and neoangiogenesis). It would be interesting if future prospective studies could focus on the relationships in selected different points of the tumour between FET-PET data, intraoperative 5-ALA data and histopathological data. This would be useful, above all, when surgery on recurrent gliomas is considered. The observation of vivid 5-ALA uptake in their case of low-grade glioma is intriguing and warrants further investigations.
Domenico d’Avella
Alessandro Della Puppa
Padova, Italy
Rights and permissions
About this article
Cite this article
Stockhammer, F., Misch, M., Horn, P. et al. Association of F18-fluoro-ethyl-tyrosin uptake and 5-aminolevulinic acid-induced fluorescence in gliomas. Acta Neurochir 151, 1377–1383 (2009). https://doi.org/10.1007/s00701-009-0462-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-009-0462-7