Abstract
Background
The combination of a neurokinin-1 receptor antagonist, dexamethasone, and a 5-HT3 receptor antagonist is currently the standard antiemetic treatment in patients receiving cisplatin-based high emetogenic chemotherapy (HEC). The aim of this study was to evaluate the efficacy of a combination of palonosetron, a unique second-generation 5-HT3 receptor antagonist, aprepitant, the only approved neurokinin-1 receptor antagonist, and dexamethasone as antiemetic prophylaxis in patients receiving HEC (cisplatin ≥50 mg/mq).
Methods
Chemotherapy-naïve adult patients, receiving cisplatin-based HEC, were treated with palonosetron 0.25 mg/i.v., dexamethasone 20 mg/i.v., and aprepitant 125 mg/p.o., 1-h before chemotherapy. Aprepitant 80 mg/p.o. and dexamethasone 4 mg p.o. were administered on days 2–3. Primary end point was complete response (CR; no vomiting and no use of rescue medication), during the overall study period (0–120 h). Secondary end points were complete control (CR and no more than mild nausea), emesis-free rate, and nausea-free rate during the acute (0–24 h), delayed (24–120 h), and overall (0-120 h) periods. Safety was also evaluated.
Results
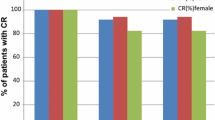
A total of 222 patients were included in the study. Median age was 62 years, 76.6% were male and 23.4% female, and most common tumors were lung (66.7%) and head and neck (15.8%); 70.3% of patients achieved CR during the overall study period. Complete control, emesis-free rate, and nausea-free rate were 70.3%, 92.8%, and 59.9%, respectively, during the overall phase. The most commonly reported side effects were constipation (39% of patients) and headache (5%).
Conclusions
This study shows that palonosetron in combination with aprepitant and dexamethasone is effective to prevent chemotherapy-induced nausea and vomiting in patients treated with cisplatin-based HEC.

Similar content being viewed by others
References
Gralla RJ, Osoba D, Kris MG et al (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American society of clinical oncology. J Clin Oncol 17:2971–2994
Trigg ME, Higa GM (2010) Chemotherapy-induced nausea and vomiting: antiemetic trials that impacted clinical practice. J Oncol Pharm Pract. doi:10.1177/1078155209354655
Hesketh PJ (1999) Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist 4:191–196
Roila F, Hesketh PJ, Herrstedt J et al (2006) Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia international antiemetic consensus conference. Ann Oncol 17:20–28
Herrstedt J (2008) Antiemetics: an update and the MASCC guidelines applied in clinical practice. Nat Clin Pract Oncol 5(1):32–43
Kris MG, Hesketh PJ, Somerfield MR et al (2006) American society of clinical oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947
Kris MG (2005) Consensus proposals for the prevention of acute and delayed vomiting and nausea following high-emetic-risk chemotherapy. Support Care Cancer 13:85–96
Forni C, Ferrari S, Loro L et al (2000) Granisetron, tropisetron, and ondansetron in the prevention of acute emesis induced by a combination of cisplatin-adriamycin and by high-dose ifosfamide delivered in multi-day continuous infusions. Support Care Cancer 8:131–133
Geling O, Eichler H-G (2005) Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol 23:1289–1294
Eisenberg P, Figuero-Vadillo J, Zamora R et al (2003) Improved prevention of moderately emetogenic chemotherapy induced nausea and vomiting with palonosetron, a pharmacologically novel 5HT3 receptor antagonist. Results of a phase III, single dose trial versus dolasetron. Cancer 98(11):2473–2482
Gralla R, Lichinitser M, Van Der Vegt S et al (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14:1570–1577
Aapro MS, Bertoli L, Lordick F et al (2003) Palonosetron (PALO) is effective in preventing acute and delayed chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Supportive Care Cancer 11(6): abstract no. A-17
Rubenstein EB, Gralla RJ, Eisenberg P et al (2003) Palonosetron (PALO) compared with ondansetron (OND) or dolasetron (DOL) for prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV). Combined results of two Phase III trials. Proc Am Soc Clin Oncol 22(729):A2932
Saito M, Aogi K, Sekine I et al (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10(2):115–124
Rojas C, Stathis M, Thomas AG et al (2008) Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg 107:469–478
Rojas C, Thomas AG, Alt J et al (2010) Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol 626(2–3):193–199
Stoltz R, Cyong JC, Shah A et al (2004) Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in U.S. and Japanese healthy subjects. J Clin Pharmacol 44:520–531
Wong EH, Clark R, Leung E et al (1995) The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol 114(4):851–859
Italian Group for Antiemetic Research (1998) Double-blind, dose-finding study of four intravenous doses of dexamethasone in the prevention of cisplatin-induced acute emesis. J Clin Oncol 16:2937–2942
Grunberg SM (2007) Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: dosing, efficacy, and tolerability analysis. Ann Oncol 18:233–240
Vardy JL, Chiew K, Galica J et al (2006) Side effects associated with dexamethasone for prophylaxis of delayed emesis after moderately emetogenic therapy. Br J Cancer 94:1011–1015
Curran MP, Robinson DM (2009) Aprepitant: a review of its use in the prevention of nausea and vomiting. Drugs 69(13):1853–1878
Grote T, Hajdenberg J, Cartmell A et al (2006) Combination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: palonosetron, dexamethasone and aprepitant. J Support Oncol 4:403–408
Grunberg SM, Dugan M, Muss H et al (2009) Effectiveness of a single-day three-drug regimen of dexamethasone, palonosetron, and aprepitant for the prevention of acute and delayed nausea and vomiting caused by moderately emetogenic chemotherapy. Support Care Cancer 17(5):589–594
Herrington JD, Jaskiewicz AD, Song J (2008) Randomized, placebo-controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. Cancer 112(9):2080–2087
Hesketh PJ, Kris MG, Grunberg SM et al (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 15:103–109
Aapro M, Fabi A, Nolè F et al (2010) Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol. doi:10.1093/annonc/mdp584
Celio L, Bajetta E, Denaro A et al (2009) Single-day regimen of palonosetron (PALO) and dexamethasone (DEX) for the prevention of emesis associated with moderately emetogenic chemotherapy (MEC): subgroup analysis from a randomized phase III trial. J Clin Oncol 27:15s (suppl; abstr 9620)
Hesketh PJ, Grunberg SM, Gralla RJ et al (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomised, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin: the aprepitant protocol 052 study group. J Clin Oncol 21(22):4112–4119
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD et al (2003) Addition of the neurokinin 1 receptor anatagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting: results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97(12):3090–3098
Schmoll HJ, Aapro MS, Poli-Bigelli S et al (2006) Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 17(6):1000–1006
Rubenstein EB, Slusher BS, Rojas C et al (2006) New approaches to chemotherapy-induced nausea and vomiting: from neuropharmacology to clinical investigations. Cancer J 12(5):341–347
Tattersall FD, Rycroft W, Francis B et al (1996) Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacol 35:1121–1129
Sanger GJ (1992) The involvement of 5-HT3 receptors in visceral function. In: Hamon M (ed) Central and peripheral 5-HT3 receptors. Academic Press, London, pp 207–255
Heitz P, Polak JM, Timson DM et al (1976) Enterochromaffin cells as the endocrine source of gastrointestinal substance P. Histochemistry 49:343–347
Hu WP, You XH, Guan BC et al (2004) Substance P potentiates 5-HT3 receptor-mediated current in rat trigeminal ganglion neurons. Neurosci Lett 365(2):147–152
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Longo, F., Mansueto, G., Lapadula, V. et al. Palonosetron plus 3-day aprepitant and dexamethasone to prevent nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 19, 1159–1164 (2011). https://doi.org/10.1007/s00520-010-0930-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-010-0930-x