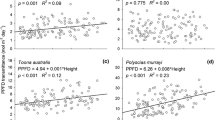
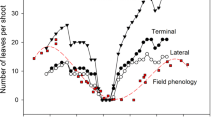
Abstract
Saplings of many canopy tree species in winter deciduous forests receive the major portion of their light budget for their growing season prior to canopy closure in the spring. This period of high light may be critical for achieving a positive carbon (C) gain, thus contributing strongly to their growth and survival. This study of saplings of Aesculus glabra and Acer saccharum in Trelease Woods, Illinois, USA, tested this hypothesis experimentally by placing tents of shade cloth over saplings during their spring period of high light prior to canopy closure in three consecutive years. Leaf senescence began 16 days (year 0) and 60 days (year 1) earlier for shaded A. glabra saplings than control saplings. No change in senescence occurred for A. saccharum. The annual absolute growth in stem diameter of both species was negligible or negative for shaded saplings, but positive for control saplings. Only 7% of the shaded A. glabra saplings were alive after 2 years, while all control saplings survived for 3 years; only 20% of the shaded A. saccharum saplings survived for 3 years, while 73% of control saplings were alive after the same period. Early spring leaf out is a critical mechanism that allows the long-term persistence of saplings of these species in this winter deciduous forest. Studies and models of C gain, growth, and survival of saplings in deciduous forests may need to take into account their spring phenology because saplings of many species are actually “sun” individuals in the spring prior to their longer period in the summer shade.



Similar content being viewed by others
References
Abrams MD, Kubiske ME (1990) Leaf structural characteristics of 31 hardwood and conifer tree species in central Wisconsin USA: influence of light regime and shade-tolerance rank. For Ecol Manage 31:245–254
Augspurger CK (2004) Developmental vs environmental control of early leaf phenology in juvenile Ohio buckeye (Aesculus glabra). Can J Bot 82:31–35
Augspurger CK, Bartlett EA (2003) Differences in leaf phenology between juvenile and adult trees in a temperate deciduous forest. Tree Physiol 23:517–526
Augspurger CK, Cheeseman JM, Salk CF (2005) Light gains and physiological capacity of understorey woody plants during phenological avoidance of canopy shade. Funct Ecol 19:537–546
Baker FS (1949) A revised shade tolerance table. J For 47:179–181
Burns RM, Honkala BH (1990) Silvics of North America. Hardwoods, vol 2. USDA Handbook 654. U.S. Department of Agriculture, Washington D.C.
Canham CD, Kobe RK, Latty EF, Chazdon RL (1999) Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121:1–11
Casperson JP, Kobe RK (2001) Interspecific variation in sapling mortality in relation to growth and soil moisture. Oikos 92:160–168
Chazdon RL (1988) Sunflecks and their importance to forest understory plants. Adv Ecol Res 18:1–63
Ellsworth JW, Harrington RA, Fownes JH (2004) Survival, growth and gas exchange of Celastrus orbiculatus seedlings in sun and shade. Am Mid Nat 151:233–240
Gaucher C, Gougeon S, Mauffette U, Messier C (2005) Seasonal variation in biomass and carbohydrate partitioning of understorey sugar maple (Acer saccharum) and yellow birch (Betula alleghaniensis) seedlings. Tree Physiol 25:93–100
Gill DS, Amthor JS, Bormann FH (1998) Leaf phenology, photosynthesis, and the persistence of saplings and shrubs in a mature northern hardwood forest. Tree Physiol 18:281–289
Givnish TJ (ed) (1986) On the economy of plant form and function. Cambridge University Press, New York
Gross K, Homlicher A, Weinreich A, Wagner E (1996) Effect of shade on stomatal conductance, net photosynthesis, photochemical efficiency and growth of oak seedlings. Ann Sci For 53:279–290
Harrington RA, Brown BJ, Reich PB (1989) Ecophysiology of exotic and native shrubs in Southern Wisconsin 1 Relationship of leaf characteristics, resource availability, and phenology to seasonal patterns of carbon gain. Oecologia 80:356–367
Jolly WM, Nemani R, Running SW (2004) Enhancement of understory productivity by asynchronous phenology with overstory competitors in a temperate deciduous forest. Tree Physiol 24:1069–1071
Jones RH, Allen BP, Sharitz RR (1997) Why do early-emerging tree seedlings have survival advantage? A test using Acer rubrum (Aceraceae). Am J Bot 84:1714–1718
Kobe RK (1997) Carbohydrate allocation to storage as a basis of interspecific variation in sapling survivorship and growth. Oikos 80:226–233
Kobe RK, Pacala SW, Silander JA Jr, Canham CD (1995) Juvenile tree survivorship as a component of shade tolerance. Ecol Appl 5:517–532
Pacala SW, Canham CD, Silander JA Jr, Kobe RK (1994) Sapling growth as a function of resources in a north temperate forest. Can J For Res 24:2172–2183
dePamphilis CW, Neufeld HS (1989) Phenology and ecophysiology of Aesculus sylvatica, a vernal understorey tree. Can J Bot 67:2161–2167
Poorter L (2007) Are species adapted to their regeneration niche, adult niche, or both? Am Nat 169:433–442
Rothstein DE, Zak DR (2001) Photosynthetic adaptation and acclimation to exploit seasonal periods of direct irradiance in three temperate, deciduous-forest herbs. Funct Ecol 15:722–731
Seiwa K (1998) Advantages of early germination for growth and survival of seedlings of Acer mono under different overstorey phenologies in deciduous broad-leaved forests. J Ecol 86:219–228
Uemura S (1994) Patterns of leaf phenology in forest understorey. Can J Bot 72:409–414
Walters MB, Reich PB (1999) Low-light carbon balance and shade tolerance in the seedlings of woody plants: Do winter deciduous and broad-leaved evergreen species differ? New Phytol 143:143–154
Acknowledgments
The author thanks Sabrina Russo and Yiching Lin for help in setting up the tents, Steve Buck for managing the area, and John Cheeseman for determining light transmission of the shade cloth. The manuscript benefitted from constructive comments of C. Canham and R. Kobe. The experiment complies with the current laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Peter Reich.
Rights and permissions
About this article
Cite this article
Augspurger, C.K. Early spring leaf out enhances growth and survival of saplings in a temperate deciduous forest. Oecologia 156, 281–286 (2008). https://doi.org/10.1007/s00442-008-1000-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1000-7