Abstract
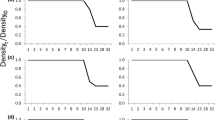
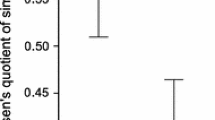
Populations of species with complex life cycles have the potential to be regulated at multiple life history stages. However, research tends to focus on single stage density-dependence, which can lead to inaccurate conclusions about population regulation and subsequently hinder conservation efforts. In amphibians, many studies have demonstrated strong effects of larval density and have often assumed that populations are regulated at this life history stage. However, studies examining density regulation in the terrestrial stages are rare, and the functional relationships between terrestrial density and vital rates in amphibians are unknown. We determined the effects of population density on survival, growth and reproductive development in the terrestrial stage of two amphibians by raising juvenile wood frogs (Rana sylvatica) and American toads (Bufo americanus) at six densities in terrestrial enclosures. Density had strong negative effects on survival, growth and reproductive development in both species. We fitted a priori recruitment functions to describe the relationship between initial density and the density of survivors after one year, and determined the functional relationship between initial density and mass after one year. Animals raised at the lowest densities experienced growth and survival rates that were over twice as great as those raised at the highest density. All female wood frogs in the lowest density treatment showed signs of reproductive development, compared to only 6% in the highest density treatment. Female American toads reached minimum reproductive size only at low densities, and male wood frogs and American toads reached maturity only in the three lowest density treatments. Our results demonstrate that in the complex life cycle of amphibians, density in the terrestrial stage can reduce growth, survival and reproductive development and may play an important role in amphibian population regulation. We discuss the implications of these results for population regulation in complex life cycles and for amphibian conservation.







Similar content being viewed by others
References
Akaike H (1992) Information theory and an extension of the maximum likelihood principle. In: Kotz S, Johnson N (eds) Breakthroughs in statistics. Springer, Heidelberg, pp 610–624
Altwegg (2003) Multistage density dependence in an amphibian. Oecologia 136:46–50
Beck CW, Congdon JD (1999) Effects of individual variation in age and size at metamorphosis on growth and survivorship of southern toad (Bufo bufo) metamorphs. Can J Zool 77:944–951
Beebee TJC, Denton JS, Buckley J (1996) Factors affecting population densities of adult natterjack toads Bufo calamita in Britain. J Appl Ecol 33:263–268
Benton TG, Grant A (1999) Elasticity analysis as an important tool in evolutionary and population ecology. Trends Ecol Evol 14:467–471
Berven KA (1982) The genetic basis of altitudinal variation in the wood frog Rana sylvatica. II. An experimental analysis of larval development. Oecologia 52:360–369
Berven KA (1990) Factors affecting population fluctuation in larval and adult stages of the wood frog (Rana sylvatica). Ecology 71:1599–1608
Berven KA (1995) Population regulation in the wood frog, Rana sylvatica from three diverse geographic localities. Aust J Ecol 20:385–392
Beverton RJH, Holt SJ (1957) On the dynamics of exploited fish populations. Fish Invest (Lond) 2:1–533
Biek R, Funk WC, Maxell BA, Mills LS (2002) What is missing in amphibian decline research: insights from ecological sensitivity analysis. Conserv Biol 16:728–734
Cohen MP, Alford RA (1993) Growth, survival and activity patterns of recently metamorphosed Bufo marinus. Wildl Res 20:1–13
Crouse D, Crowder L, Caswell H (1987) A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology 68:1412–1423
Di Fiore MM, Baccari GC, Rastogi RK, di Meglio M, Pinelli C, Iela L (2005) Hormonal regulation of secondary sexual characters. In: Heatwole H (ed) Amphibian biology, vol 6: endocrinology. Surrey Beatty and Sons, Chipping Norton, NSW, Australia, pp 2228–2249
Gamble LR, McGarigal K, Jenkins CL, Timm BC (2006) Limitations of regulated “buffer zones” for the conservation of marbled salamanders. Wetlands 26:298–306
Gill DE (1979) Density dependence and homing behavior in adult red-spotted newts Notophthalmus viridescens (Rafinesque). Ecology 60:800–813
Gittins SP (1983) Population dynamics of the common toad (Bufo bufo) at a lake in Mid-Wales. J Anim Ecol 52:981–988
Halpern BS, Gaines SD, Warner RR (2005) Habitat size, recruitment, and longevity as factors limiting population size in stage-structured species. Am Nat 165:82–94
Heatwole H (1961) Habitat selection and activity of the wood frog, Rana sylvatica Le Conte. Am Midl Nat 66:301–313
Hellriegel B (2000) Single- or multistage regulation in complex life cycles: does it make a difference? Oikos 88:239–249
Henle K, Sarre S, Wiegand K (2004) The role of density regulation in extinction processes and population viability analysis. Biodiver Conser 13:9–52
Howard RD (1980) Mating behavior and mating success in woodfrogs, Rana sylvatica. Anim Behav 28:705–716
James SM (2005) Amphibian metamorphosis and juvenile terrestrial performance following chronic cadmium exposure in the aquatic environment. Ph.D. Thesis, University of Missouri-Columbia, CO, pp 197
Jorgensen CB (1992) Growth and reproduction. In: Feder ME, Burggren WW (eds) Environmental physiology of the amphibians. University of Chicago, Chicago, IL, pp 439–466
Kalb HJ, Zug GR (1990) Age estimates for a population of American toads, Bufo americanus (Salientia: Bufonidae), in northern Virginia. Brimleyana 16:79–86
Marsh DM (2001) Fluctuations in amphibian populations: a meta-analysis. Biol Conserv 101:327–335
Morris WF, Doak DF (2002) Quantitative conservation biology. Sinauer Associates, Sunderland, MA
Nelder JA (1961) The fitting of a generalization of the logistic curve. Biometrics 17:89–110
Newman RA (1987) Effects of density and predation on Scaphiopus couchii tadpoles in desert ponds. Oecologia 71:301–307
Patrick DA (2007) The effects of forest practices on a Maine amphibian community. Ph.D. dissertation, Dept. Wildlife Ecology, University of Maine, Orono, pp 119
Patrick DA, Hunter MLJ, Calhoun AJK (2006) Effects of experimental forestry treatments on a Maine amphibian community. For Ecol Manage 234:323–332
Pechmann JHK (1994) Population regulation in complex life cycles: aquatic and terrestrial density-dependence in pond-breeding amphibians. Ph.D. Thesis, Department of Zoology, Duke University, Durham, NC, pp 146
Pfister CA (1998) Patterns of variance in stage-structured populations: evolutionary predictions and ecological implications. Proc Nat Acad Sci USA 95:213–218
Pinder AW, Storey KB, Ultsch GR (1992) Estivation and hibernation. In: Feder ME, Burggren WW (eds) Environmental physiology of the amphibians. University of Chicago, Chicago, IL, pp 250–276
Regosin JV, Windmiller BS, Homan RN, Reed JM (2005) Variation in terrestrial habitat use by four poolbreeding amphibian species. J Wildl Manage 69:1481–1493
Regosin JV, Windmiller BS, Reed JM (2003) Terrestrial habitat use and winter densities of the Wood frog (Rana sylvatica). J Herpetol 37:390–394
Ricker WE (1954) Stock and recruitment. J Fish Res Board Can 11:559–623
Rittenhouse TAG, Semlitsch RD (2007) Distribution of amphibians in terrestrial habitat surrounding wetlands. Wetlands 27:153–161
Roberts W, Lewin V (1979) Habitat utilitzation and population densities of the amphibians of northeastern Alberta. Can Field Nat 93:144–154
Rodriguez DJ (1988) Models with density regulation in more than one life stage. Theor Popul Biol 34:93–117
Rothermel BB, Semlitsch RD (2002) An experimental investigation of landscape resistance of forest versus old-field habitats to emigrating juvenile amphibians. Conserv Biol 16:1324–1332
Rothermel BB, Semlitsch RD (2006) Consequences of forest fragmentation for juvenile survival in spotted (Ambystoma maculatum) and marbled (A. opacum) salamanders. Can J Zool 84:797–807
Sayler A (1966) The reproductive ecology of the red-backed salamander, Plethodon cinereus, in Maryland. Copeia 1966:183–193
Schmid WD (1965) Some aspectes of the water economies of nine species of amphibians. Ecology 46:261–269
Scott DE, Fore MR (1995) The effect of food limitation on lipid levels, growth, and reproduction in the marbled salamander, Ambystoma opacum. Herpetologica 51:462–471
Semlitsch RD (1981) Terrestrial activity and summer home range of the mole salamander (Ambystoma talpoideum). Can J Zool 59:315–322
Semlitsch RD (ed)(2003) Amphibian conservation, 1st edn. Smithsonian Institution Press, Washington, DC
Semlitsch RD, Bodie JR (2003) Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv Biol 17:1219–1228
Semlitsch RD, Scott DE, Pechmann JHK, Gibbons JW (1996) Structure and dynamics of an amphibian community: evidence from a 16-year study of a natural pond. In: Cody ML, Smallwood JA (eds) Long-term studies of vertebrate communities. Academic, San Diego, CA, pp 217–248
Shepherd JG (1982) A versatile new stock recruitment relationship for fisheries, and the construction of sustainable yield curves. J Cons Int Explor Mer 40:67–75
Shoop CR (1974) Yearly variation in larval survival of Ambystoma maculatum. Ecology 55:440–444
Skelly DK, Kiesecker JM (2001) Venue and outcome in ecological experiments: manipulations of larval anurans. Oikos 94:198–208
Smith DC (1983) Factors controlling tadpole populations of the chorus frog (Pseudacris triseriata) on Isle Royale, Michigan. Ecology 64:501–510
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
Taylor BE, Scott DE (1997) Effects of larval density dependence on population dynamics of Ambystoma opacum. Herpetologica 53:132–145
Trenham PC, Shaffer HB (2005) Amphibian upland habitat use and its consequences for population viability. Ecol Appl 15:1158–1168
Trenham PC, Shaffer HB, Koenig WD, Stromberg MR (2000) Life history and demographic variation in the California tiger salamander (Ambystoma californiense). Copeia 2000:365–377
VanBuskirk J, Smith DC (1991) Density-dependent population regulation in a salamander. Ecology 72:1747–1756
Vasconcelos D, Calhoun AJK (2004) Movement patterns of adult and juvenile Rana sylvatica (LeConte) and Ambystoma maculatum (Shaw) in three restored seasonal pools in Maine. J Herpetol 38:551–561
Vonesh JR, De la Cruz O (2002) Complex life cycles and density dependence: assessing the contribution of egg mortality to amphibian declines. Oecologia 133:325–333
Vucetich JA, Waite TA, Qvarnemark L, Ibargüen S (2000) Population variability and extinction risk. Conserv Biol 14:1704–1714
White GC, Burnham KP (1999) Program MARK: Survival estimation from populations of marked animals. Bird Stud 46:S120–S138
Wilbur HM (1980) Complex life cycles. Ann Rev Ecol Syst 11:67–93
Acknowledgments
We thank T. Altnether, D. Patrick, L. Rehard, and T. Rittenhouse for their assistance in the field. C. Galen, D. Patrick, T. Rittenhouse, B. Rothermel, J. Vonesh and two anonymous reviewers provided useful comments on earlier drafts of the manuscript. This research was supported by a grant from the National Science Foundation DEB 0239943. Eggs and larvae were collected under Missouri Department of Conservation Wildlife Collector’s permits 12,227 and 12,696, and maintained under University of Missouri Animal Care and Use Protocol 3368.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Craig Osenberg.
Rights and permissions
About this article
Cite this article
Harper, E.B., Semlitsch, R.D. Density dependence in the terrestrial life history stage of two anurans. Oecologia 153, 879–889 (2007). https://doi.org/10.1007/s00442-007-0796-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0796-x