Abstract
The unimodal, right-skewed distribution, most frequently identified in contemporary descriptions of placental mammal body size distributions, masks an underlying multidistribution structure; a long-term evolutionary process that has generated a concatenation of two or three frequency distributions specific to locomotory modes (plantigrade, digitigrade and unguligrade). The Afrotropical assemblages are bimodal, with a tendency towards trimodality, whereas the Nearctic assemblage is unimodal. However, mixtures of two and three normal distributions fitted the Nearctic data well, suggesting a multidistribution structure masked by disproportionate species numbers within locomotory modes. Differences in proportional species numbers within modes between assemblages may reflect the evolutionary history of form and function. However, common interassemblage predictions of such proportions in contemporary distributions may be disguised by the relative severity of the Pleistocene megafaunal extinction (patterns supported by the fossil record), geographical scale, and taxonomic composition. A species gap occurs at body sizes around 1 kg at the interface between the largest plantigrade mammals and the smallest digitigrade mammals, coincident with the minimum interspecific variance of basal metabolic rate. In terms of the evolution of the optimal body size in the trade-off between mortality and production, there may be good historical and evolutionary reasons why we should not expect optimization to produce the same results in different zoogeographical assemblages. Moreover, the evolution of diverse mammalian forms and functions, especially with respect to predator-prey interactions and diet, render a single body size optimum untenable in the search for an energetic definition of fitness.






Similar content being viewed by others
References
Adkins RM, Gelke EL, Rowe D, Honeycutt RL (2001) Molecular phylogeny and divergence time estimates for major rodent groups: evidence from multiple genes. Mol Biol Evol 18:777–791
Alroy J (1998) Cope’s Rule and the dynamics of body mass evolution in North American fossil mammals. Science 280:731–734
Benton MJ (2000) Vertebrate palaeontology. Blackwell, London
Bininda-Emonds ORP, Gittleman JL, Purvis A (1999) Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia). Biol Rev 74:143–175
Blackburn TM, Gaston KJ (1994) Animal body size distributions: patterns, mechanisms and implications. Trends Ecol Evol 9:471–474
Blackburn TM, Gaston KJ (1996) On being the right size: different definitions of ‘right’. Oikos 75:551–557
Blackburn TM, Gaston KJ (1998) The distribution of mammal body masses. Divers Distrib 4:121–133
Bokma F (2001) Evolution of body size: limitations of an energetic definition of fitness. Funct Ecol 15:696–699
Bowman AW, Azzalini A (1997) Applied smoothing techniques for data analysis. Clarendon, Oxford
Brown JH, Maurer BA (1986) Body size, ecological dominance and Cope’s rule. Nature 324:248–250
Brown JH, Nicoletto PF (1991) Spatial scaling of species composition: body masses of North American land mammals. Am Nat 138:1478–1512
Brown JH, Marquet PA, Taper ML (1993) Evolution of body size: consequences of an energetic definition of fitness. Am Nat 142:573–584
Burt WH, Grossenheider RP (1976) A field guide to the mammals of America north of Mexico. Houghton Mifflin, Boston
Calow P (1977) Ecology, evolution and energetics: a study in metabolic adaptation. Adv Ecol Res 10:1–62
Choquenot D, Bowman DMJS (1998) Marsupial megafauna, aborigines and the overhill hypothesis: application of predator-prey models to the question of Pleistocene extinction in Australia. Global Ecol Biog 7:167–180
Chown SL, Gaston KJ (1997) The species-body size distribution: energy, fitness and optimality. Funct Ecol 11:365–375
Damuth J (1981) Population density and body size in mammals. Nature 290:699–700
Demment MW, Van Soest PJ (1985) A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. Am Nat 125:641–672
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman & Hall, New York
Eizirik E, Murphy WJ, Obrien SJ (2001) Molecular dating and biogeography of the early placental mammal radiation. J Hered 92:212–219
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Flannery T (1994) The future eaters. Braziller, New York
Flannery TF, Gott B (1985) The Spring Creek locality: a late Pleistocene megafaunal site from southwestern Victoria. Aust Zool 21:385–422
Garland T (1983a) Scaling the ecological cost of transport to body mass in terrestrial mammals. Am Nat 121:571–587
Garland T (1983b) The relation between maximal running speed and body mass in terrestrial mammals. J Zool (Lond) 199:157–170
Garland T, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41:18–32
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford
Harvey PH, Pagel MD, Rees JA (1991) Mammalian metabolism and life histories. Am Nat 137:556–566
Hildebrand M (1974) Analysis of vertebrate structure. Wiley, New York
Holling CS (1992) Cross-scale morphology, geometry, and dynamics of ecosystems. Ecol Monogr 62:447–502
Huchon D, Douzery EJP (2001) From the old world to the new world: a molecular chronicle of the phylogeny and biogeography of Hystricognath rodents. Mol Phylogenet Evol 20:238–251
Jones KE, Purvis A (1997) An optimum body size for mammals? Comparative evidence from bats. Funct Ecol 11:751–756
Kindlmann P, Dixon AFG, Dostalkova I (1999) Does body size optimization result in skewed body size distribution on a logarithmic scale? Am Nat 153:445–447
Kingdon J (1997) The Kingdon field guide to African mammals. Academic, London
Klein RG (1984) Southern African prehistory and paleoenvironments. Balkema, Boston
Kozłowski J (1996a) Optimal initial size and adult size of animals: consequences for macroevolution and community structure. Am Nat 147:101–114
Kozłowski J (1996b) Energetic definition of fitness? Yes, but not that one. Am Nat 147:1087–1091
Kozłowski J, Gawelczyk AT (2002) Why are species’ body size distributions usually skewed to the right? Funct Ecol 16:419–432
Kozłowski J, Weiner J (1997) Interspecific allometries are the by-products of body size optimization. Am Nat 149:352–380
Kumar S, Hedges SB (1998) A molecular timescale for vertebrate evolution. Nat 392:917–920
Kurtén B (1971) The age of mammals. Trinity, London
Loder N, Blackburn TM, Gaston KJ (1997) The slippery slope: towards an understanding of the body size frequency distribution. Oikos 78:195–201
Lomolino MV (1985) Body size of animals on islands: the island rule reexamined. Am Nat 125:310–316
Lovegrove BG (2000) The zoogeography of mammalian basal metabolic rate. Am Nat 156:201–219
Lovegrove BG (2001) The evolution of body armor in mammals: plantigrade constraints of large body size. Evolution 55:1464–1473
Maglio VJ, Cooke HBS (1978) Evolution of African mammals. Harvard University Press, Massachusetts
Manly BFJ (1996) Are there clumps in body size distributions? Ecology 77:81–86
Maree S (2002) Phylogenetic relationships and mitochondrial DNA sequence evolution in the African rodent subfamily Otomyinae (Muridae). University of Pretoria, Pretoria, South Africa
Mares MA (1985) Mammal faunas of xeric habitats and the Great American Interchange. In: Stehli F, Webb D (eds) The great American biotic interchange. Plenum, New York, pp 489–520
Mares MA (1992) Neotropical mammals and the myth of Amazonian biodiversity. Science 255:976–979
Marquet PA, Taper ML (1998) On size and area: patterns of mammalian body size extremes across landmasses. Evol Ecol 12:127–139
Marquet PA, Navarrete SA, Castilla JC (1995) Body size, population density, and the energetic equivalence rule. J Anim Ecol 64:325–332
Marshall LG (1984) Who killed cock robin: an investigation of the extinction controversy. In: Martin PS, Klein RG (eds) Quaternary extinctions: a prehistoric revolution. University of Arizona Press, Tucson, Ariz., pp 785–806
Martin PS (1984) Prehistoric overkill: the global model. In: Martin PS, Klein RG (eds) Quaternary extinctions, a prehistoric revolution. University of Arizona Press, Tucson, Ariz., pp 354–403
Matthee CA, Robinson TJ (1999) Cytochrome b phylogeny of the family Bovidae: resolution within the Alcephini, Antilopini, Neotragini, and Tragelaphini. Mol Phylogenet Evol 12:31–46
Maurer BA, Brown JH, Rusler RD (1992) The micro and macro in body size evolution. Evolution 46:939–953
Michaux J, Reyes A, Catzeflis F (2001) Evolutionary history of the most speciose mammals: molecular phylogeny of muroid rodents. Mol Biol Evol 18:2017–2031
Montgelard C, Bentz S, Tirard C, Verneau O, Catzeflis FM (2002) Molecular systematics of Sciurognathi (Rodentia): the mitochondrial cytochrome b and 12S rRNA genes support the Anomaluroidea (Pedetidae and Anomaluridae). Mol Phylogenet Evol 22:220–233
Murphy WJ, Eizirik E, Johnson WE, Zhang YP, Ryderk OA, Obrien SJ (2001) Molecular phylogenetics and the origins of placental mammals. Nature 409:614–618
Nowak RM, Paradiso JL (1983) Walker’s mammals of the world. Johns Hopkins University Press, Baltimore
Owen-Smith N (1988) Megafaunal extinctions: the conservation message from 11,000 years B.P. Conserv Biol 3:405–412
Pagel M (1992) A method for the analysis of comparative data. J Theor Biol 156:431–442
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Querouil S, Hutterer R, Barriere P, Colyn M, Peterhans JCK, Verheyen E (2001) Phylogeny and evolution of African shrews (Mammalia:Soricidae) inferred from 16 s rRNA sequences. Mol Phylogenet Evol 20:185–195
Qumsiyeh MB (1986) Phylogenetic studies of the rodent family Gerbillidae: 1. Chromosomal evolution of the southern African complex. J Mamm 67:680–692
Qumsiyeh MB, Hamilton MJ, Dempster ER, Baker RJ (1991) Cytogenetics and systematics of the rodent genus Gerbillurus. J Mamm 72:89–96
Raman J, Perrin MR (1997) Allozyme and isozyme variation in seven southern African elephant–shrew species. Z Saeugetierkd 62:108–116
Romer AS (1966) Vertebrate palaeontology. University of Chicago Press, Chicago
Siemann E, Brown JH (1999) Gaps in mammalian body size distributions reexamined. Ecology 80:2788–2792
Silva M, Downing JA (1995) CRC handbook of mammalian body masses. CRC Press, Boca Raton
Silverman BW (1981) Using kernel density estimates to investigate multimodality. J R Stat Soc B 43:97–99
Silverman BW (1986) Density estimation for statistics and data analysis. Chapman & Hall, New York
Simpson GG (1961) Horses. Anchor, New York
Simpson GG (1980) Splendid isolation: the curious history of South American mammals. Yale University Press, New Haven
Skinner JD, Smithers RHN (1990) The mammals of the southern African subregion. University of Pretoria, Pretoria, South Africa
Springer MS, Cleven GC, Madsen O, de Jong WW, Waddell VG, Amrine HM, Stanhope MJ (1997) Endemic African mammals shake the phylogenetic tree. Nature 388:61–64
Stanley SM (1973) An explanation for Cope’s rule. Evolution 27:1–26
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Valen L van(1973) Body size and numbers of plants and animals. Evolution 27:27–35
Walton AH, Nedbal MA, Honeycutt RL (2000) Evidence from intron 1 of the nuclear transthyretin (Prealbumin) gene for the phylogeny of African mole-rats (Bathyergidae). Mol Phylogenet Evol 16:467–474
Acknowledgements
Grants from the NRF and the University of Natal Research Fund to B.G.L. financed this research. The authors are very grateful to Steven Chown for comments on the draft manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Clade I
Topology
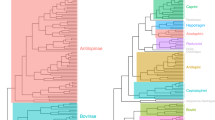
Orders (Eizirik et al. 2001), Afrosoricidae (Chrysochloridae)(G. Bronner, personal communication), Macroscelidea (Raman and Perrin 1997) (Fig. 7).
Divergence dates
Node A, 102.1 mya (Eizirik et al. 2001); node B, 85 mya; node C, 79.9 mya; node D, 54.8 mya (Springer et al. 1997); node E, 24.3 mya (Eizirik et al. 2001); node G, 23 mya (Benton 2000). Node F was placed arbitrarily equidistant between nodes C and E. All tip branch lengths were arbitrarily set to 2 mya. All remaining branch lengths were chosen arbitrarily . The outgroup (marsupial) branch lengths are not to scale.
Clade III
Topology
Hystricognaths and Sciurognaths (Montgelard et al. 2002), Muridae (Michaux et al. 2001), Otomyinae (Maree 2002), Bathyergidae (Walton et al. 2000), Gerbillidae (Qumsiyeh 1986; Qumsiyeh et al. 1991) (Fig. 8).
Divergence dates
Node A, 75.1 mya (Adkins et al. 2001); node B, 45.7 mya; node C, 51.2 mya; node D, 28.9 mya (Montgelard et al. 2002); node E, 18.8 mya; node F, 9 mya; node G, 13.3 mya; node H, 9 mya; node I, 10.5 mya (Michaux et al. 2001); node J, 5 mya (Maree 2002); node K, 28 mya; node L, 48 mya; node M, 63 mya; node N, 11 mya (Huchon and Douzery 2001). Nodes O, P, Q and R were placed arbitrarily 2, 4, 6 and 8 mya earlier than node A, respectively. All tip branch lengths were arbitrarily set to 2 mya and all remaining branches of unknown length were chosen arbitrarily.
Clade IV
Topology
Bovidae (Matthee and Robinson 1999), Carnivora (Bininda-Emonds et al. 1999), Eulipotyphla (Querouil et al. 2001) (Fig. 9).
Divergence dates
Node A, 93.2 mya (mean estimate Eizirik et al. 2001); node G, 93.9 mya, and node I, 94.6 mya, were placed equidistant between the maximum estimate of the primate-clade IV divergence (Node F, 95.3 mya in Fig. 2) and node A. Node B, 64.7 mya (Kumar and Hedges 1998); node C, 55 mya; node J, 38 mya (Benton 2000); node D, 22.3 mya (Matthee and Robinson 1999); node H, 53.8 mya (Bininda-Emonds et al. 1999); node I, 96.3 mya (see clade 1); node K, 19.1 mya (Querouil et al. 2001). Nodes E and F were placed arbitrarily equidistant between nodes B and D.
Rights and permissions
About this article
Cite this article
Lovegrove, B.G., Haines, L. The evolution of placental mammal body sizes: evolutionary history, form, and function. Oecologia 138, 13–27 (2004). https://doi.org/10.1007/s00442-003-1376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1376-3