Abstract
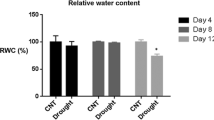
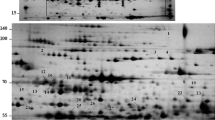
Resurrection plants differ from other species in their unique ability to survive desiccation. In order to understand the mechanisms of desiccation tolerance, proteome studies were carried out using leaves of the resurrection plant Boea hygrometrica to reveal proteins that were differentially expressed in response to changes in relative water content. This opportunity was afforded by the rare ability of excised B. hygrometrica leaves to survive and resume metabolism following desiccation in a manner similar to intact plants. From a total of 223 proteins that were reproducibly detected and analyzed, 35% showed increased abundance in dehydrated leaves, 5% were induced in rehydrated leaves and 60% showed decreased or unchanged abundance in dehydrated and rehydrated leaves. Since the induction kinetics fall into clearly defined patterns, we suggest that programmed regulation of protein expression triggered by changes of water status. Fourteen dehydration responsive proteins were analyzed by mass spectrometry. Eight proteins were classified as playing a role in reactive oxygen species scavenging, photosynthesis and energy metabolism. In agreement with these findings, glutathione content and polyphenol oxidase activity were found to increase upon dehydration and rapid recovery of photosynthesis was observed.








Similar content being viewed by others
References
Alamillo JM, Bartels D (2001) Effects of desiccation on photosynthesis pigments and the ELIP-like dsp 22 protein complexes in the resurrection plant Craterostigma plantagineum. Plant Sci 160:1161–1170
Albert VA, Williams SE, ChaseMW (1992) Carnivorous plants: phylogeny and structural evolution. Science 257:1491–1495
Augusti A, Scartazza A, Navari-Izzo F, Sgherri CLM, Stevanović B, Brugnoli E (2001) Photosystem II photochemical efficiency, zeaxanthin and antioxidant contents in the poikilohydric Ramonda serbica during dehydration and rehydration. Photosynth Res 67:79–88
Baier M, Dietz KJ (1998) The costs and benefits of oxygen in photosynthetic plant metabolism. Prog Bot 60:283–314
Bartels D, Salamini F (2001) Desiccation tolerance in the resurrection plant Craterostigma plantagineum. A contribution to the study of drought tolerance at the molecular level. Plant Physiol 127:1346–1353
Bartels D, Schneider K, Terstappen G, Piatkowski D, Salamini F (1990) Molecular cloning of abscissic acid-modulated genes which are induced during desiccation of the resurrection plant Craterostigma plantagineum. Planta 181:27–34
Bernacchia G, Schwall G, Lottspeich F, Salamini F, Bartels D (1995) The transketolase gene family of the resurrection plant Craterostigma plantagineum differential expression during the rehydration phase, EMBO J 14:610–618
Bianchi G, Gamba A, Murelli C, Salamini F, Bartels D (1992) Low molecular weight solutes in desiccated and ABA-treated calli and leaves of Craterostigma plantagineum. Phytochemistry 31:1917–1922
Blomstedt CK, Gianello RD, Hamill JD, Neale AD, Gaff DF (1998a) Drought-stimulated genes correlated with desiccation tolerance of the resurrection grass Sporobolus stapfianus. Plant Growth Regul 24:153–161
Blomstedt CK, Gianello RD, Gaff DF, Hamill JD, Neale AD (1998b) Differential gene expression in desiccation-tolerant and desiccation-sensitive tissue of the resurrection grass, Sporobolus stapfianus. Aust J Plant Physiol 25:937–946
Bochicchio A, Vazzana C, Puliga S, Alberti A, Cinganelli S, Vernieri P (1998) Moisture content of the dried leaf is critical to desiccation tolerance in detached leaves of the resurrection plant Boea hygroscopica. Plant Growth Regul 24:163–170
Bowler C, van Montague M, Inzé D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Churin Y, Schilling S, Borner T (1999) A gene family encoding glutathione peroxidase homologues in Hordeum vulgare (barley). FEBS Lett 459:33–38
Collett H, Butowt R, Smith J, Farrant J, Illing N (2003) Photosynthetic genes are differentially transcribed during the dehydrationrehydration cycle in the resurrection plant, Xerophyta humilis. J Exp Bot 54:2593–2595
Collett H, Shen A, Gardner M, Farrant JM, Denby KJ, Illing N (2004) Towards transcript profiling of desiccation tolerance in Xerophyta humilis: Construction of a normalized 11 k X. humilis cDNA set and microarray expression analysis of 424 cDNAs in response to dehyration. Physiol Plant 122:39–53
Cooper K, Farrant JM (2002) Recovery of the resurrection plant Craterostigma wilmsii from desiccation: protection versus repair. J Exp Bot 53:1805–1813
Dai S, Li L, Chen T, Chong K, Xue Y, Wang T (2006) Proteomic analyses of Oryza sativa mature pollen reveal novel proteins associated with pollen germination and tube growth. Proteomics 6:2504–2529
Damerval C, Vienne D, Zivy M, Thiellement H (1986) The technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 7:52–54
Deng X, Hu Z, Wang H (1999) mRNA differential display visualized by silver staining tested on gene expression in resurrection plant Boea hygrometrica. Plant Mol Biol Rep 17:279
Deng X, Hu Z, Wang H, Wen X, Kuang T (2003) A comparison of photosynthetic apparatus of the detached leaves of the resurrection plant Boea hygrometrica with its non-tolerant relative Chirita heterotrichia in response to dehydration and rehydration. Plant Sci 165:851–861
Desimone M, Wagner E, Johanningmeier U (1998) Degradation of active oxygen modified Rubisco by chloroplastic proteases requieres ATP-hydrolysis. Planta 205:459–466
Farrant JM (2000) A comparison of mechanisms of desiccation tolerance among three angiosperm resurrection plant species. Plant Ecol 151:29–39
Farrant J, Cooper K, Kruger LA, Sherwin HW (1999) The effect of rate of drying on three different resurrection plants. Ann Bot 84:371–379
Farrant JM, Vander Willigen C, Loffell DA, Bartsch S, Whittaker A (2003) An investigation into the role of light during desiccation of three angiosperm resurrection plants. Plant Cell Environ 26:1275–1286
Futcher B, Latter GI, Monardo P, McLaughlin CS, Garrels JI (1999) A sampling of the yeast proteome. Mol Cell Biol 19:7357–7368
Gaff DF (1971) Desiccation-tolerant flowering plants in Southern Africa. Science 25:1033–1034
Gaff DF (1997) Mechanism of desiccation tolerance in resurrection vascular plants. In: Basra AS, Basra RK (eds) Mechanisms of environmental stress resistance in plants. Harwood Academic, Amsterdam, pp 43–58
Gaff DF, Loveys BR (1984) Abscisic acid content and effects during dehydration ofdetached leaves of desiccation tolerant plants. J Exp Bot 35:1350–1358
Gaff DF, Bartels D, Gaff JL (1997) Changes in gene expression duringdrying in a desiccation-tolerant grass Sporobolus stapfianus and a desiccation-sensitive grass Sporobolus pyramidalis. Aust J Plant Physiol 24:617–622
Gygi SP, Rochon Y, Franza BR, Aebersold R (1999) Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 19:1720–1730
Hajheidari M, Abdollahian-Noghabi M, Askari H, Heidari1 M, Sadeghian S, Ober E, Salekdeh G (2005) Proteome analysis of sugar beet leaves under drought stress. Proteomics, 5:950–960
Hamilton CA, Good AG, Taylor GJ (2001) Induction of vacuolar ATPase and mitochondrial ATP synthase by aluminum in an aluminum-resistant cultivar of wheat. Plant Physiol 125:2068–2077
Hamilton CA, Taylor GJ, Good AG (2002) Vacuolar H+-ATPase, but not mitochondrial F1F0-ATPase, is required for NaCl tolerance in Saccharomyces cerevisiae. FEMS Microbiol Lett 208:227–232
Han G, Li S, Tang C, Li L, Kang T (2004) Progress on the structure of photosynthetic oxygen evolving complex and the mechanism of photosynthetic water oxidation. Prog Chem 16:184–195
Hellwege EM, Dietz KJ, Volk OH, Hartung W (1994) Abscisic acid and the induction of desiccation tolerance in the extremely xerophilic liverwort Exormotheca holstii. Planta 194:525–531
Higgins CF (1992) ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8:67–113
Illing N, Denby K, Collett H, Shen A, Farrant JM (2005) The signature of seeds in resurrection plants: a molecular and physiological comparison of desiccation tolerance in seeds and vegetative tissues. Integr Comp Biol 45:771–787
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403
Kamo M, Kawakami T, Miyatake N, Tsugita A (1995) Separation and characterization of Arabidopsis thaliana proteins by two-dimensional gel electrophoresis. Electrophoresis 16:423–430
Kawamura Y, Arakawa K, Maeshima M, Yoshida S (2001) ATP analogue binding to the A subunit induces conformational changes in the E subunit that involves a disulfide bond formation in plant V-ATPase. Eur J Biochem 268:2801–2809
Klein M, Perfus-Barbeoch L, Frelet A, Gaedeke N, Reinhardt D, Mueller-Roeber B, Martinoia E, Forestier C (2003) The plant multidrug resistance ABC transporter AtMRP5 is involved in guard cell hormonal signalling and water use. Plant J 33:119–129
Klein M, Geisler M, Suh S, Kolukisaoglu HÜ, Azevedo L, Plaza S, Curtis MD, Richter A, Weder B, Schulz B, Martinoia E (2004) Disruption of AtMRP4, a guard cell plasma membrane ABCC-type ABC transporter, leads to deregulation of stomatal opening and increased drought susceptibility. Plant J 39:219–236
Kuang J, Gaff DF, Gianello RD, Blomstedt CK, Neale AD, Hamill JD. (1995) Changes in in vivo protein complements in drying leaves of the desiccation-tolerant grass Sporobolus stapfianus and the desiccation-sensitive grass Sporobolus pyramidalis. Aust J Plant Physiol 22:1027–1034
Larsen PB, Geisler MJ, Jones CA, Williams KM, Cancel JD (2005) ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J 41:353–363
Marais S, Thomson JA, Farrant JM, Mundree SG (2004) VATP1XV—a novel stress responsive V-ATPase subunit c homologue isolated from the resurrection plant Xerophyta viscosa Baker. Physiol Plant 122:54–61
Monk LS, Fagerstedt KV, Crawford RMM (1989) Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiol Plant 76:456–459
Mundree SG, Baker B, Mowla S, Peters S, Marais S, Vander Willigen C, Govender K, Maredza A, Muyanga S, Farrant JM, Thomson JA (2002) Physiological and molecular insights into drought tolerance. Afri J Biotechnol 1:28–38
Navari-Izzo F, Meneguzzo S, Loggini B, Vazzana C, Sgherri CLM (1997) The role of the glutathione system during dehydration of Boea hygroscopica. Physiol Plant 99:23–30
Neale AD, Blomstedt CK, Bronson P, Le TN, Gutteridge K, Evans J, Gaff DF, Hamill JD (2000) The isolation of genes from the resurrection grass Sporobolus stapfianus, which are induced during severe drought stress. Plant Cell Environ 25:265–277
Neubauer C, Yamamoto HY (1994) Membrane barriers and Mehler-peroxidase reaction limit the ascorbate available for violaxanthin de-epoxidase activity in intact chloroplasts. Photosynth Res 39:137–147
O’Farrell PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
Oliver MJ (1991) Influence of protoplasmic water loss on the control of protein synthesis in the desiccation-tolerant moss Tortula ruralis: Ramifications for a repair-based mechanism of desiccation tolerance. Plant Physiol 97:1501–1511
Oliver MJ, Tuba Z, Mishler BD (2000) The evolution of vegetative desiccation tolerance in land plants. Plant Ecol 151:85–100
Phillips JR, Oliver MJ, Bartels D (2002) Molecular genetics of desiccation tolerant systems. In: Black M, Pritchard HW (eds) Desiccation and survival in plants: drying without dying. CABI Publishing, Wallingford, pp 319–341
Ramanjulu S, Bartels D (2002) Drought- and desiccation-induced modulation of gene expression in plants. Plant Cell Environ 25:141–151
Ratajczak R (2000) Structure, function and regulation of the plant vacuolar H(+)-translocating ATPase. Biochim Biophys Acta 1465:17–36
Reynolds TL, Bewley JD (1993) Abscisic acid enhances the ability of the desiccation-tolerant fern Polypodium virginianum to withstand drying. J Exp Bot 44:1771–1779
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Roxas VP, Smith RK, Allen ER, Allen RD (1997) Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat Biotechnol 15:988–991
Rohrig H, Schmidt J, Colby T, Brautigam A, Hufnagel P, Bartels D (2006) Desiccation of the resurrection plant Craterostigma plantagineum induces dynamic changes in protein phosphorylation. Plant Cell Environ 29:1606–1617
Salekdeh GH, Siopongco J, Wade LJ, Ghareyazie B, Bennett J (2002) Proteomic analysis of rice leaves during drought stress and recovery. Proteomics 2:1131–1145
Sedmak JJ, Grossberg SE (1977) A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem 79:544–552
Seidler A (1996) The extrinsic polypeptides of Photosystem II. Biochim Biophys Acta 1277:35–60
Sgherri CLM, Navari-Izzo F (1995) Sunflower seedlings subjected to increasing water deficit stress: oxidative stress and defence mechanisms. Physiol Plant 93:25–30
Sherman TD, Gardeur TL, Lax AR (1995) Implications of the phyogenetic distribution of polyphenol oxidase in plants. In: Lee CY, Whitaker JR (eds) Enzymatic browning and its prevention. American Chemical Society, Washington, DC, pp 103–119
Sherwin HW (1995) Desiccation tolerance and sensitivity of vegetative plant tissue. PhD thesis, University of Natal, Durban, South Africa
Sherwin HW, Farrant JM (1998) Protection mechanism against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Regul 24:203–210
Smith-Espinoza CJ (2001) Analysis of ABA and drought stress mediated gene expression in the desiccation tolerant resurrection plant Craterostigma plantagineum. PhD thesis, University of Cologne, Germany
Sullivan ML, Hatfield RD (2006) Polyphenol oxidase and o-diphenols inhibit post-harvest proteolysis in red clover and alfalfa. Crop Physiol Met 46:662–670
Sze H, Li XH, Palmgren MG (1999) Energization of plant cell membranes by H+-pumping ATPases. Regulation biosynthesis. Plant Cell 11:677–689
Tuba Z, Proctor MCF, Csintalan Zs (1998) Ecophysiological responses of homoichlorophyllous and poikilichlorophyllous desiccation tolerant plants: a comparison and an ecological perspective. Plant Growth Regul 24:211–217
Tuba Z, Smirnoff N, Csintalan Zs, Nagy Z, Szente K (1997) Respiration during slow desiccation of the poikilochlorophyllous desiccation tolerant plant Xerophyta scabrida at present-day CO2 concentrations. Plant Physiol Biochem 35:381–386
Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11:1195–1206
Veljovic-Jovanovic S, Kukavica B, Stevanovic B, Navari-Izzo F (2006) Senescence- and drought-related changes in peroxidase and superoxide dismutase isoforms in leaves of Ramonda serbica. J Exp Bot 57:1759–1768
Vicré M, Lerouxel O, Farrant J, Lerouge P, Driouich A (2004) Composition and desiccation-induced alterations of the cell wall in the resurrection plant Craterostigma wilmsii. Physiol Plant 120:229–239
Wang Y, Tian S, Xu Y, Qin G, Yao H (2004) Changes in the activities of pro- and anti-oxidant enzymes in peach fruit inoculated with Cryptococcus laurentii or Penicillium expansum at 0 or 20°C. Postharvest Biol Technol 34:21–28
Watson BS, Asirvatham VS, Wang L, Sumner LW (2003) Mapping the proteome of Barrel Medic (Medicago truncatula). Plant Physiol 131:1104–1123
Xiong L, Zhu J (2002) Plant responses to osmotic stress. Plant Cell Environ 25:131–139
Yang P, Liang Y, Shen S, Kuang T (2006) Proteome analysis of rice uppermost internodes at the milky stage. Proteomics 6:3330–3338
Acknowledgments
We thank Dr. Shihua Shen, Institute of Botany, Chinese Academy of Sciences and Ms. Anne Bräutigam, Max-Planck-Institut für Züchtungsforschung, Germany, for their excellent discussion and aids on establishment of proteomic platform, and Professor Dr. Dorothea Bartels, IMBIO (Molekulare Physiologie und Biotechnologie der Pflanzen), University of Bonn for critical reading and valuable suggestions. The project was supported by National Natural Science Fundation of China (no. 30400027) and National Basic Research Program of China (973 Programs) (no. 2006CB403206).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jiang, G., Wang, Z., Shang, H. et al. Proteome analysis of leaves from the resurrection plant Boea hygrometrica in response to dehydration and rehydration. Planta 225, 1405–1420 (2007). https://doi.org/10.1007/s00425-006-0449-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0449-z