Abstract
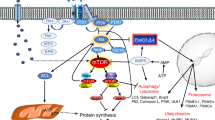
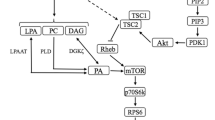
Gain or loss of skeletal muscle mass occurs in situations of altered use such as strength training, aging, denervation, or immobilization. This review examines our current understanding of the cellular and molecular events involved in the control of muscle mass under conditions of muscle use and disuse, with particular attention to the effects of resistance exercise/training. The DNA content, which is a critical determinant of protein synthesis by providing the amount of DNA necessary to sustain gene transcription, can be either increased (activation of satellite cells) or decreased (apoptosis) depending on muscle activity and ongoing physiological processes. In addition, several transcription factors are sensitive to functional demand and may control muscle-specific protein expression to promote or repress myofiber enlargement. The control of skeletal muscle mass is also markedly mediated by the regulation of transduction pathways that promote the synthesis and/or the degradation of proteins. Insulin-like growth factor-I plays a key role in this balance by activating the Akt/tuberous sclerosis complex 2/mammalian target of rapamycin pathway. Stimulation of this pathway leads to the concomitant activation of initiation and elongation factors resulting in the elevation of protein translation and the downregulation of ubiquitin proteasome components through Forkhead-box O transcription factors.





Similar content being viewed by others
References
Adams GR, Caiozzo VJ, Haddad F, Baldwin KM (2002) Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 283:C1182–C1195
Adams GR, Haddad F (1996) The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol 81:2509–2516
Adams GR, McCue SA (1998) Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84:1716–1722
Allen DL, Linderman JK, Roy RR, Grindeland RE, Mukku V, Edgerton VR (1997) Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J Appl Physiol 83:1857–1861
Allen DL, Yasui W, Tanaka T, Ohira Y, Nagaoka S, Sekiguchi C, Hinds WE, Roy RR, Edgerton VR (1996) Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J Appl Physiol 81:145–151
Allen RE, Boxhorn LK (1989) Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138:311–315
Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM (1995) Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165:307–312
Alway SE, Martyn JK, Ouyang J, Chaudhrai A, Murlasits ZS (2003) Id2 expression during apoptosis and satellite cell activation in unloaded and loaded quail skeletal muscles. Am J Physiol Regul Integr Comp Physiol 284:R540–R549
Appell HJ, Duarte JA, Soares JM (1997) Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med 18:157–160
Argiles JM, Busquets S, Lopez-Soriano FJ (2003) Cytokines in the pathogenesis of cancer cachexia. Curr Opin Clin Nutr Metab Care 6:401–406
Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H (2005) Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J 19:786–788
Awede B, Thissen J, Gailly P, Lebacq J (1999) Regulation of IGF-I, IGFBP-4 and IGFBP-5 gene expression by loading in mouse skeletal muscle. FEBS Lett 461:263–267
Baar K, Esser K (1999) Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 276:C120–C127
Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM (2007) Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol 102:2232–2239
Banzet S, Koulmann N, Sanchez H, Serrurier B, Peinnequin A, Bigard AX (2007) Musclin gene expression is strongly related to fast-glycolytic phenotype. Biochem Biophys Res Commun 353:713–718
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Barton ER (2006) Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol 100:1778–1784
Berthon P, Duguez S, Favier FB, Amirouche A, Feasson L, Vico L, Denis C, Freyssenet D (2007) Regulation of ubiquitin-proteasome system, caspase enzyme activities, and extracellular proteinases in rat soleus muscle in response to unloading. Pflugers Arch 454:625–633
Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR (2005) Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J Appl Physiol 98:482–488
Bickel CS, Slade JM, Haddad F, Adams GR, Dudley GA (2003) Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol 94:2255–2262
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Booth FW, Seider MJ (1979) Early change in skeletal muscle protein synthesis after limb immobilization of rats. J Appl Physiol 47:974–977
Brack AS, Bildsoe H, Hughes SM (2005) Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci 118:4813–4821
Busquets S, Figueras MT, Meijsing S, Carbo N, Quinn LS, Almendro V, Argiles JM, Lopez-Soriano FJ (2005) Interleukin-15 decreases proteolysis in skeletal muscle: a direct effect. Int J Mol Med 16:471–476
Cai D, Frantz JD, Tawa NE Jr., Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119:285–298
Carbo N, Lopez-Soriano J, Costelli P, Busquets S, Alvarez B, Baccino FM, Quinn LS, Lopez-Soriano FJ, Argiles JM (2000) Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br J Cancer 83:526–531
Carson JA, Booth FW (1998) Myogenin mRNA is elevated during rapid, slow, and maintenance phases of stretch-induced hypertrophy in chicken slow-tonic muscle. Pflugers Arch 435:850–858
Carson JA, Schwartz RJ, Booth FW (1996) SRF and TEF-1 control of chicken skeletal alpha-actin gene during slow-muscle hypertrophy. Am J Physiol 270:C1624–C1633
Carson JA, Wei L (2000) Integrin signaling’s potential for mediating gene expression in hypertrophying skeletal muscle. J Appl Physiol 88:337–343
Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B (2006) Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech Ageing Dev 127:794–801
Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA (2006) Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 20:190–192
Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, Grizard J, Attaix D (2005) A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J Physiol 569:489–499
Day MK, Allen DL, Mohajerani L, Greenisen MC, Roy RR, Edgerton VR (1995) Adaptations of human skeletal muscle fibers to spaceflight. J Gravit Physiol 2:P47–P50
Degens H, Alway SE (2006) Control of muscle size during disuse, disease, and aging. Int J Sports Med 27:94–99
Desvigne N, Barthélémy J, Frère D, Gay-Montchamp J, Costes F (2007) Insulin-like growth factor-I kinetic in human muscle interstitial fluid after resistance exercise. Sci Sports 22:117–119
Duguez S, Bihan MC, Gouttefangeas D, Feasson L, Freyssenet D (2003) Myogenic and nonmyogenic cells differentially express proteinases, Hsc/Hsp70, and BAG-1 during skeletal muscle regeneration. Am J Physiol Endocrinol Metab 285:E206–E215
Dunn SE, Burns JL, Michel RN (1999) Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem 274:21908–21912
Dunn SE, Chin ER, Michel RN (2000) Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J Cell Biol 151:663–672
Dupont-Versteegden EE, Fluckey JD, Knox M, Gaddy D, Peterson CA (2006) Effect of flywheel-based resistance exercise on processes contributing to muscle atrophy during unloading in adult rats. J Appl Physiol 101:202–212
Dupont-Versteegden EE, Knox M, Gurley CM, Houle JD, Peterson CA (2002) Maintenance of muscle mass is not dependent on the calcineurin-NFAT pathway. Am J Physiol Cell Physiol 282:C1387–C1395
Durieux AC, Amirouche A, Banzet S, Koulmann N, Bonnefoy R, Pasdeloup M, Mouret C, Bigard X, Peinnequin A, Freyssenet D (2007) Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Endocrinology 148:3140–3147
Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR (1996) Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol 270:E627–E633
Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW (1999) Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol 277:C152–C162
Fluckey JD, Knox M, Smith L, Dupont-Versteegden EE, Gaddy D, Tesch PA, Peterson CA (2006) Insulin-facilitated increase of muscle protein synthesis after resistance exercise involves a MAP kinase pathway. Am J Physiol Endocrinol Metab 290:E1205–E1211
Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V (2003) Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell 12:51–62
Funai K, Parkington JD, Carambula S, Fielding RA (2006) Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 290:R1080–R1086
Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N (2002) FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress. J Biol Chem 277:26729–26732
Galvagni F, Lestingi M, Cartocci E, Oliviero S (1997) Serum response factor and protein-mediated DNA bending contribute to transcription of the dystrophin muscle-specific promoter. Mol Cell Biol 17:1731–1743
Gan B, Yoo Y, Guan JL (2006) Association of focal adhesion kinase with tuberous sclerosis complex 2 in the regulation of s6 kinase activation and cell growth. J Biol Chem 281:37321–37329
Gilson H, Schakman O, Combaret L, Lause P, Grobet L, Attaix D, Ketelslegers JM, Thissen JP (2007) Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 148:452–460
Goldberg AL (1967) Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol 213:1193–1198
Goldberg AL (1968) Protein synthesis during work-induced growth of skeletal muscle. J Cell Biol 36:653–658
Goldspink DF (1976) The effects of denervation on protein turnover of rat skeletal muscle. Biochem J 156:71–80
Goldspink DF (1977) The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol 264:267–282
Goldspink DF, Morton AJ, Loughna P, Goldspink G (1986) The effect of hypokinesia and hypodynamia on protein turnover and the growth of four skeletal muscles of the rat. Pflugers Arch 407:333–340
Gordon SE, Fluck M, Booth FW (2001) Selected contribution: skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol 90:1174–1183
Greenhalgh CJ, Bertolino P, Asa SL, Metcalf D, Corbin JE, Adams TE, Davey HW, Nicola NA, Hilton DJ, Alexander WS (2002) Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b). Mol Endocrinol 16:1394–1406
Gundersen K, Merlie JP (1994) Id-1 as a possible transcriptional mediator of muscle disuse atrophy. Proc Natl Acad Sci USA 91:3647–3651
Haar EV, Lee SI, Bandhakavi S, Griffin TJ, Kim DH (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9:316–323
Haddad F, Adams GR (2004) Inhibition of MAP/ERK kinase prevents IGF-I-induced hypertrophy in rat muscles. J Appl Physiol 96:203–210
Haddad F, Adams GR (2006) Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol 100:1188–1203
Haddad F, Baldwin KM, Tesch PA (2005) Pretranslational markers of contractile protein expression in human skeletal muscle: effect of limb unloading plus resistance exercise. J Appl Physiol 98:46–52
Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM (2003) Atrophy responses to muscle inactivity. II. Molecular markers of protein deficits. J Appl Physiol 95:791–802
Haddad F, Zaldivar F, Cooper DM, Adams GR (2005) IL-6-induced skeletal muscle atrophy. J Appl Physiol 98:911–917
Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD (2003) Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547:247–254
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M (2006) Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and -tendon: Differential effects of specific contraction types. J Appl Physiol 102:573–581
Hespel P, Op’t Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA (2001) Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol 536:625–633
Hoppeler H (1986) Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 7:187–204
Hornberger TA, Hunter RB, Kandarian SC, Esser KA (2001) Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats. Am J Physiol Cell Physiol 281:C179–C187
Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA (2004) Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 380:795–804
Hunter RB, Kandarian SC (2004) Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest 114:1504–1511
Ikemoto M, Nikawa T, Takeda S, Watanabe C, Kitano T, Baldwin KM, Izumi R, Nonaka I, Towatari T, Teshima S, Rokutan K, Kishi K (2001) Space shuttle flight (STS-90) enhances degradation of rat myosin heavy chain in association with activation of ubiquitin-proteasome pathway. FASEB J 15:1279–1281
Johnson TL, Klueber KM (1991) Skeletal muscle following tonic overload: functional and structural analysis. Med Sci Sports Exerc 23:49–55
Jones SW, Hill RJ, Krasney PA, O’Conner B, Peirce N, Greenhaff PL (2004) Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J 18:1025–1027
Judge AR, Koncarevic A, Hunter RB, Liou HC, Jackman RW, Kandarian SC (2007) Role for IkappaBalpha, but not c-Rel, in skeletal muscle atrophy. Am J Physiol Cell Physiol 292:C372–C382
Kadi F, Bonnerud P, Eriksson A, Thornell LE (2000) The expression of androgen receptors in human neck and limb muscles: effects of training and self-administration of androgenic-anabolic steroids. Histochem Cell Biol 113:25–29
Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, Olsen S, Kjaer M (2005) The behaviour of satellite cells in response to exercise: what have we learned from human studies. Pflugers Arch 451:319–327
Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL (2004) The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558:1005–1012
Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O (2004) Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279:41114–41123
Kandarian SC, Jackman RW (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33:155–165
Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C (2004) Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci USA 101:18135–18140
Kim JS, Cross JM, Bamman MM (2005) Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288:E1110–E1119
Koncarevic A, Jackman RW, Kandarian SC (2007) The ubiquitin-protein ligase Nedd4 targets Notch1 in skeletal muscle and distinguishes the subset of atrophies caused by reduced muscle tension. FASEB J 21:427–437
Kraemer WJ (1988) Endocrine responses to resistance exercise. Med Sci Sports Exerc 20:S152–S157
Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS (2005) Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem 280:7570–7580
Kvorning T, Andersen M, Brixen K, Schjerling P, Suetta C, Madsen K (2007) Suppression of testosterone does not blunt mRNA expression of myoD, myogenin, IGF, myostatin or androgen receptor post strength training in humans. J Physiol 578:579–593
Lalani R, Bhasin S, Byhower F, Tarnuzzer R, Grant M, Shen R, Asa S, Ezzat S, Gonzalez-Cadavid NF (2000) Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J Endocrinol 167:417–428
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18:39–51
Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE (2005) Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 288:R1288–R1296
Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP (2006) Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576:923–933
Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB (1998) Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J 12:871–880
Loughna PT, Brownson C (1996) Two myogenic regulatory factor transcripts exhibit muscle-specific responses to disuse and passive stretch in adult rats. FEBS Lett 390:304–306
Lowe DA, Lund T, Alway SE (1998) Hypertrophy-stimulated myogenic regulatory factor mRNA increases are attenuated in fast muscle of aged quails. Am J Physiol 275:C155–C162
Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP (2005) Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121:179–193
MacDougall JD, Tarnopolsky MA, Chesley A, Atkinson SA (1992) Changes in muscle protein synthesis following heavy resistance exercise in humans: a pilot study. Acta Physiol Scand 146:403–404
Machida S, Booth FW (2004) Regrowth of skeletal muscle atrophied from inactivity. Med Sci Sports Exerc 36:52–59
McCarthy JJ, Esser KA (2007) MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol 102:306–313
McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162:1135–1147
McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209:501–514
McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G (1999) Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol 516(Pt 2):583–592
McMahon CD, Popovic L, Oldham JM, Jeanplong F, Smith HK, Kambadur R, Sharma M, Maxwell L, Bass JJ (2003) Myostatin-deficient mice lose more skeletal muscle mass than wild-type controls during hindlimb suspension. Am J Physiol Endocrinol Metab 285:E82–E87
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Mitchell PO, Mills ST, Pavlath GK (2002) Calcineurin differentially regulates maintenance and growth of phenotypically distinct muscles. Am J Physiol Cell Physiol 282:C984–C992
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215–228
Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ (2005) Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 288:E1153–E1159
Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200
Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N (1999) IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400:581–585
Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN (2000) Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem 275:4545–4548
Nicholas G, Thomas M, Langley B, Somers W, Patel K, Kemp CF, Sharma M, Kambadur R (2002) Titin-cap associates with, and regulates secretion of, Myostatin. J Cell Physiol 193:120–131
Odelberg SJ, Kollhoff A, Keating MT (2000) Dedifferentiation of mammalian myotubes induced by msx1. Cell 103:1099–1109
Olson EN, Brennan TJ, Chakraborty T, Cheng TC, Cserjesi P, Edmondson D, James G, Li L (1991) Molecular control of myogenesis: antagonism between growth and differentiation. Mol Cell Biochem 104:7–13
Owino V, Yang SY, Goldspink G (2001) Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett 505:259–263
Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD (2004) Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem 279:26192–26200
Perrone CE, Fenwick-Smith D, Vandenburgh HH (1995) Collagen and stretch modulate autocrine secretion of insulin-like growth factor-1 and insulin-like growth factor binding proteins from differentiated skeletal muscle cells. J Biol Chem 270:2099–2106
Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM (2006) Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291:E937–E946
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273:E99–E107
Pistilli EE, Siu PM, Alway SE (2006) Interleukin-15 responses to aging and unloading-induced skeletal muscle atrophy. Am J Physiol Cell Physiol 292:C1298–C1304
Puri PL, Sartorelli V, Yang XJ, Hamamori Y, Ogryzko VV, Howard BH, Kedes L, Wang JY, Graessmann A, Nakatani Y, Levrero M (1997) Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell 1:35–45
Rao PK, Kumar RM, Farkhondeh M, Baskerville S, Lodish HF (2006) Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci USA 103:8721–8726
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S (2006) Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 101:53–59
Reardon KA, Davis J, Kapsa RM, Choong P, Byrne E (2001) Myostatin, insulin-like growth factor-1, and leukemia inhibitory factor mRNAs are upregulated in chronic human disuse muscle atrophy. Muscle Nerve 24:893–899
Reid MB (2005) Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol 288:R1423–R1431
Reynolds TH IV, Bodine SC, Lawrence JC Jr (2002) Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem 277:17657–17662
Riechman SE, Balasekaran G, Roth SM, Ferrell RE (2004) Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol 97:2214–2219
Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL (1994) Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761–771
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013
Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA (2003) Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood) 228:706–709
Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Metter EJ, Hurley BF, Rogers MA (2001) Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol A Biol Sci Med Sci 56:B240–B247
Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL (2007) Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21:140–155
Sakamoto K, Aschenbach WG, Hirshman MF, Goodyear LJ (2003) Akt signaling in skeletal muscle: regulation by exercise and passive stretch. Am J Physiol Endocrinol Metab 285:E1081–E1088
Sakuma K, Nakao R, Inashima S, Hirata M, Kubo T, Yasuhara M (2004) Marked reduction of focal adhesion kinase, serum response factor and myocyte enhancer factor 2C, but increase in RhoA and myostatin in the hindlimb dy mouse muscles. Acta Neuropathol (Berl) 108:241–249
Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103:16260–16265
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Schmalbruch H, Lewis DM (2000) Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve 23:617–626
Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM (1999) Skeletal muscle hypertrophy is mediated by a Ca2+ -dependent calcineurin signalling pathway. Nature 400:576–581
Serrano AL, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lomo T, Schiaffino S (2001) Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc Natl Acad Sci USA 98:13108–13113
Servais S, Letexier D, Favier R, Duchamp C, Desplanches D (2007) Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis. Free Radic Biol Med 42:627–635
Seward DJ, Haney JC, Rudnicki MA, Swoap SJ (2001) bHLH transcription factor MyoD affects myosin heavy chain expression pattern in a muscle-specific fashion. Am J Physiol Cell Physiol 280:C408–C413
Sinha-Hikim I, Roth SM, Lee MI, Bhasin S (2003) Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 285:E197–E205
Siu PM, Pistilli EE, Alway SE (2005) Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol 289:R1015–R1026
Smith HK, Maxwell L, Martyn JA, Bass JJ (2000) Nuclear DNA fragmentation and morphological alterations in adult rabbit skeletal muscle after short-term immobilization. Cell Tissue Res 302:235–241
Southgate RJ, Neill B, Prelovsek O, El-Osta A, Kamei Y, Miura S, Ezaki O, McLoughlin TJ, Zhang W, Unterman TG, Febbraio MA (2007) FOXO1 regulates the expression of 4E-BP1 and inhibits mTOR signaling in mammalian skeletal muscle. J Biol Chem 282:21176–21186
Spangenburg EE, Abraha T, Childs TE, Pattison JS, Booth FW (2003) Skeletal muscle IGF-binding protein-3 and -5 expressions are age, muscle, and load dependent. Am J Physiol Endocrinol Metab 284:E340–E350
Staron RS, Leonardi MJ, Karapondo DL, Malicky ES, Falkel JE, Hagerman FC, Hikida RS (1991) Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol 70:631–640
Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC (2003) Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol 551:33–48
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Sucharov CC, Mariner P, Long C, Bristow M, Leinwand L (2003) Yin Yang 1 is increased in human heart failure and represses the activity of the human alpha-myosin heavy chain promoter. J Biol Chem 278:31233–31239
Sutrave P, Leferovich JM, Kelly AM, Hughes SH (2000) The induction of skeletal muscle hypertrophy by a ski transgene is promoter-dependent. Gene 241:107–116
Taaffe DR, Jin IH, Vu TH, Hoffman AR, Marcus R (1996) Lack of effect of recombinant human growth hormone (GH) on muscle morphology and GH-insulin-like growth factor expression in resistance-trained elderly men. J Clin Endocrinol Metab 81:421–425
Thomason DB, Booth FW (1990) Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol 68:1–12
Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA (2005) Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem 280:2847–2856
Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A (1995) Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol 269:E820–E826
Vandenburgh H, Kaufman S (1980) Protein degradation in embryonic skeletal muscle. Effect of medium, cell type, inhibitors, and passive stretch. J Biol Chem 255:5826–5833
Walker KS, Kambadur R, Sharma M, Smith HK (2004) Resistance training alters plasma myostatin but not IGF-1 in healthy men. Med Sci Sports Exerc 36:787–793
Wan M, Wu X, Guan KL, Han M, Zhuang Y, Xu T (2006) Muscle atrophy in transgenic mice expressing a human TSC1 transgene. FEBS Lett 580:5621–5627
Wang H, Hertlein E, Bakkar N, Sun H, Acharyya S, Wang J, Carathers M, Davuluri R, Guttridge DC (2007) NF-kappaB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Mol Cell Biol 27:4374–4387
Wang X, Blagden C, Fan J, Nowak SJ, Taniuchi I, Littman DR, Burden SJ (2005) Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev 19:1715–1722
Wehling M, Cai B, Tidball JG (2000) Modulation of myostatin expression during modified muscle use. FASEB J 14:103–110
Welle S, Bhatt K, Shah B, Thornton C (2002) Insulin-like growth factor-1 and myostatin mRNA expression in muscle: comparison between 62–77 and 21–31 yr old men. Exp Gerontol 37:833–839
Wing SS, Haas AL, Goldberg AL (1995) Increase in ubiquitin-protein conjugates concomitant with the increase in proteolysis in rat skeletal muscle during starvation and atrophy denervation. Biochem J 307(Pt 3):639–645
Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D (1999) Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96:7324–7329
Yang SY, Goldspink G (2002) Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522:156–160
Yang Y, Creer A, Jemiolo B, Trappe S (2005) Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98:1745–1752
Yarasheski KE, Zachwieja JJ, Bier DM (1993) Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol 265:E210–E214
Yimlamai T, Dodd SL, Borst SE, Park S (2005) Clenbuterol induces muscle-specific attenuation of atrophy through effects on the ubiquitin-proteasome pathway. J Appl Physiol 99:71–80
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Favier, F.B., Benoit, H. & Freyssenet, D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflugers Arch - Eur J Physiol 456, 587–600 (2008). https://doi.org/10.1007/s00424-007-0423-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-007-0423-z