Abstract
Purpose
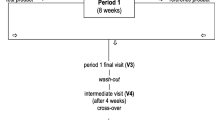
To determine the effect of 4 weeks of supplementation, then, withdrawal of a dietary supplement (DS) containing red yeast rice extract, policosanol and artichoke leaf extract at twice the recommended daily dose (6 tablets, 6-TAB) compared to the usual dose (3-TAB) or to a placebo (PLA), on blood lipid profiles and safety biomarkers.
Methods
Forty-five healthy subjects (15 per group), with untreated hypercholesterolaemia, were included in this randomised, double-blind, placebo-controlled clinical trial.
Results
After 4 weeks of supplementation, LDL-C was significantly lower in 6-TAB (−0.21 g/l; 95 % CI −0.38 to −0.03 g/l; p = 0.0217) and 3-TAB (−0.25 g/l; 95 % CI −0.42 to −0.07 g/l; p = 0.0071) compared to PLA, although no difference in LDL-cholesterol was observed between the two groups, while no effect was seen on triacylglycerol and HDL-cholesterol. Four weeks after the end of supplementation, no difference in LDL-C was seen between the PLA group and the DS-treated groups. The muscle breakdown biomarkers, as well as biomarkers of liver and renal function, were altered by neither dose of the DS. Acute application of the DS on permeabilised skeletal muscle fibres of rats did not induce deleterious effects on mitochondrial function.
Conclusions
Supplementation with twice the recommended dose of the DS was effective in reducing LDL-cholesterol and appeared safe, but according to the present results, no additional benefit could be achieved compared to the recommended dose.

Similar content being viewed by others
Abbreviations
- ALE:
-
Artichoke leaf extract
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- CK:
-
Creatine kinase
- DS:
-
Dietary supplement
- HDL-C:
-
HDL-cholesterol
- LDH:
-
Lactate dehydrogenase
- LDL-C:
-
LDL-cholesterol
- PLA:
-
Placebo
- RYR:
-
Red yeast rice
- TAB:
-
Tablet
- TC:
-
Total cholesterol
- ULN:
-
Upper limit of normality
References
Reiner Z, Catapano AL, De Backer G et al (2011) ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 32(14):1769–1818
Smith SC Jr, Benjamin EJ, Bonow RO et al (2011) AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 124(22):2458–2473
Mancini GB, Baker S, Bergeron J et al (2011) Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can J Cardiol 27(5):635–662
Liu J, Zhang J, Shi Y et al (2006) Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chin Med 1:4
Lu Z, Kou W, Du B et al (2008) Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol 101(12):1689–1693
Klimek M, Wang S, Ogunkanmi A (2009) Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. Pharm Ther 34(6):313–327
Wider B, Pittler MH, Thompson-Coon J et al (2009) Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst Rev (4):CD003335
Gouni-Berthold I, Berthold HK (2002) Policosanol: clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am Heart J 143(2):356–365
Berthold HK, Unverdorben S, Degenhardt R et al (2006) Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: a randomized controlled trial. JAMA 295(19):2262–2269
Ogier N, Amiot MJ, George S et al (2012) LDL-cholesterol-lowering effect of a dietary supplement with plant extracts in subjects with moderate hypercholesterolemia. Eur J Nutr [Epub ahead of print]
Sirvent P, Mercier J, Lacampagne A (2008) New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol 8(3):333–338
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Sirvent P, Bordenave S, Vermaelen M et al (2005) Simvastatin induces impairment in skeletal muscle while heart is protected. Biochem Biophys Res Commun 338(3):1426–1434
Saks VA, Veksler VI, Kuznetsov AV et al (1998) Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 184(1–2):81–100
Jiang FY, Sun LP, Yang J (2011) Effects of xuezhikang at different doses on patients suffering from acute coronary syndrome after percutaneous coronary intervention. Zhongguo Zhong Xi Yi Jie He Za Zhi 31(12):1607–1610 (In Chinese)
Pons P, Rodriguez M, Robaina C et al (1994) Effects of successive dose increases of policosanol on the lipid profile of patients with type II hypercholesterolaemia and tolerability to treatment. Int J Clin Pharmacol Res 14(1):27–33
Crouse JR 3rd, Lukacsko P, Niecestro R et al (2002) Dose response, safety, and efficacy of an extended-release formulation of lovastatin in adults with hypercholesterolemia. Am J Cardiol 89(2):226–229
Hunninghake DB, Knopp RH, Schonfeld G et al (1990) Efficacy and safety of pravastatin in patients with primary hypercholesterolemia. I. A dose-response study. Atherosclerosis 85(1):81–89
Jacotot B, Banga JD, Pfister P et al (1994) Efficacy of a low dose-range of fluvastatin (XU 62–320) in the treatment of primary hypercholesterolaemia. A dose-response study in 431 patients. The French-Dutch Fluvastatin Study Group. Br J Clin Pharmacol 38(3):257–263
Saito Y, Goto Y, Dane A et al (2003) Randomized dose-response study of rosuvastatin in Japanese patients with hypercholesterolemia. J Atheroscler Thromb 10(6):329–336
Heber D, Yip I, Ashley JM et al (1999) Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr 69(2):231–236
Englisch W, Beckers C, Unkauf M et al (2000) Efficacy of artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung 50(3):260–265
Castano G, Fernandez L, Mas R et al (2002) Comparison of the efficacy, safety and tolerability of original policosanol versus other mixtures of higher aliphatic primary alcohols in patients with type II hypercholesterolemia. Int J Clin Pharmacol Res 22(2):55–66
Chen H, Ren JY, Xing Y et al (2009) Short-term withdrawal of simvastatin induces endothelial dysfunction in patients with coronary artery disease: a dose-response effect dependent on endothelial nitric oxide synthase. Int J Cardiol 131(3):313–320
Tomaszewski M, Stepien KM, Tomaszewska J et al (2011) Statin-induced myopathies. Pharmacol Rep 63(4):859–866
Kaufmann P, Torok M, Zahno A et al (2006) Toxicity of statins on rat skeletal muscle mitochondria. Cell Mol Life Sci 63(19–20):2415–2425
Kwak HB, Thalacker-Mercer A, Anderson EJ et al (2012) Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free Radical Biol Med 52(1):198–207
Sirvent P, Fabre O, Bordenave S et al (2012) Muscle mitochondrial metabolism and calcium signaling impairment in patients treated with statins. Toxicol Appl Pharmacol 259(2):263–268
Bouitbir J, Charles AL, Rasseneur L et al (2011) Atorvastatin treatment reduces exercise capacities in rats: involvement of mitochondrial impairments and oxidative stress. J Appl Physiol 111(5):1477–1483
Acknowledgments
The Laboratoire Lescuyer, a company commercialising the tested dietary supplement, financed this work. We would like to thank Wanda Lipski for language editing.
Conflict of interest
E.B., E.D., and S.L.P. are employees of the company Laboratoire Lescuyer. J.F.L. is the general director of the company. P.C., C.M., B.H. and M.C. are employed by Biofortis-Mérieux Nutrisciences. The companies Biofortis-Mérieux Nutrisciences and Laboratoire Lescuyer have carried out this trial as a joint venture. Y.Z. and J.M.B. have signed a consultancy agreement with Biofortis-Mérieux Nutrisciences. P.S. declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This trial has been registered at ClinicalTrials.gov as NCT01354340.
Rights and permissions
About this article
Cite this article
Barrat, E., Zaïr, Y., Sirvent, P. et al. Effect on LDL-cholesterol of a large dose of a dietary supplement with plant extracts in subjects with untreated moderate hypercholesterolaemia: a randomised, double-blind, placebo-controlled study. Eur J Nutr 52, 1843–1852 (2013). https://doi.org/10.1007/s00394-012-0486-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0486-2