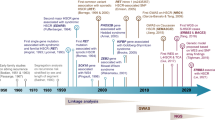
Abstract
Hirschsprung’s disease (HSCR) is a developmental disorder characterized by the absence of ganglion cells in the lower digestive tract. Aganglionosis is attributed to a disorder of the enteric nervous system (ENS) whereby ganglion cells fail to innervate the lower gastrointestinal tract during embryonic development. HSCR is a complex disease that results from the interaction of several genes and manifests with low, sex-dependent penetrance and variability in the length of the aganglionic segment. The genetic complexity observed in HSCR can be conceptually understood in light of the molecular and cellular events that take place during the ENS development. DNA alterations in any of the genes involved in the ENS development may interfere with the colonization process, and represent a primary etiology for HSCR. This review will focus on the genes known to be involved in HSCR pathology, how they interact, and on how technology advances are being employed to uncover the pathological processes underlying this disease.


Similar content being viewed by others
References
Tam PK, Garcia-Barcelo M (2004) Molecular genetics of Hirschsprung’s disease. Semin Pediatr Surg 13:236–248
Torfs C (2004) An epidemiological study of Hirschsprung’ disease in a multiracial California population. Third International Meeting: Hirschsprung’s disease and related neurocristophaties Evian, France 1998
Amiel J, Lyonnet S (2001) Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet 38:729–739
Moore SW (2006) The contribution of associated congenital anomalies in understanding Hirschsprung’s disease. Pediatr Surg Int 22:305–315
Burns AJ, Douarin NM (1998) The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development 125:4335–4347
Kruger GM, Mosher JT, Tsai YH, Yeager KJ, Iwashita T, Gariepy CE, Morrison SJ (2003) Temporally distinct requirements for endothelin receptor B in the generation and migration of gut neural crest stem cells. Neuron 40:917–929
Gariepy CE (2001) Intestinal motility disorders and development of the enteric nervous system. Pediatr Res 49:605–613
Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez R (2008) Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet 45:1–14
Swenson O (1996) Early history of the therapy of Hirschsprung’s disease: facts and personal observations over 50 years. J Pediatr Surg 31:1003–1008
Simpson NE, Kidd KK, Goodfellow PJ, McDermid H, Myers S, Kidd JR, Jackson CE, Duncan AM, Farrer LA, Brasch K (1987) Assignment of multiple endocrine neoplasia type 2A to chromosome 10 by linkage. Nature 328:528–530
Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L (1993) Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363:458–460
Grieco M, Santoro M, Berlingieri MT, Donghi R, Pierotti MA, Della PG, Fusco A, Vecchio G (1988) Molecular cloning of PTC, a new oncogene found activated in human thyroid papillary carcinomas and their lymph node metastases. Ann N Y Acad Sci 551:380–381
Grieco M, Santoro M, Berlingieri MT, Melillo RM, Donghi R, Bongarzone I, Pierotti MA, Della PG, Fusco A, Vecchio G (1990) PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 60:557–563
Donghi R, Sozzi G, Pierotti MA, Biunno I, Miozzo M, Fusco A, Grieco M, Santoro M, Vecchio G, Spurr NK (1989) The oncogene associated with human papillary thyroid carcinoma (PTC) is assigned to chromosome 10 q11-q12 in the same region as multiple endocrine neoplasia type 2A (MEN2A). Oncogene 4:521–523
Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, Howe JR, Moley JF, Goodfellow P, Wells SA Jr (1993) Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet 2:851–856
Martucciello G, Bicocchi MP, Dodero P, Lerone M, Silengo M, Puliti A, Gimelli G (1992) Total colonic aganglionosis associated with interstitial deletion of the long arm of chromosome 10. Pediatr Surg Int 7:308–310
Fewtrell MS, Tam PK, Thomson AH, Fitchett M, Currie J, Huson SM, Mulligan LM (1994) Hirschsprung’s disease associated with a deletion of chromosome 10 (q11.2q21.2): a further link with the neurocristopathies? J Med Genet 31:325–327
Angrist M, Kauffman E, Slaugenhaupt SA, Matise TC, Puffenberger EG, Washington SS, Lipson A, Cass DT, Reyna T, Weeks DE (1993) A gene for Hirschsprung disease (megacolon) in the pericentromeric region of human chromosome 10. Nat Genet 4:351–356
Lyonnet S, Bolino A, Pelet A, Abel L, Nihoul-Fekete C, Briard ML, Mok-Siu V, Kaariainen H, Martucciello G, Lerone M (1993) A gene for Hirschsprung disease maps to the proximal long arm of chromosome 10. Nat Genet 4:346–350
Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V (1995) RET-deficient mice: an animal model for Hirschsprung’s disease and renal agenesis. J Intern Med 238:327–332
Romeo G, Ronchetto P, Luo Y, Barone V, Seri M, Ceccherini I, Pasini B, Bocciardi R, Lerone M, Kaariainen H (1994) Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature 367:377–378
Edery P, Lyonnet S, Mulligan LM, Pelet A, Dow E, Abel L, Holder S, Nihoul-Fekete C, Ponder BA, Munnich A (1994) Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature 367:378–380
Brooks AS, Bertoli-Avella AM, Burzynski GM, Breedveld GJ, Osinga J, Boven LG, Hurst JA, Mancini GM, Lequin MH, de Coo RF, Matera I, de Graaff E, Meijers C, Willems PJ, Tibboel D, Oostra BA, Hofstra RM (2005) Homozygous nonsense mutations in KIAA1279 are associated with malformations of the central and enteric nervous systems. Am J Hum Genet 77:120–126
Natarajan D, Marcos-Gutierrez C, Pachnis V, de Graaff E (2002) Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development 129:5151–5160
Iwashita T, Kruger GM, Pardal R, Kiel MJ, Morrison SJ (2003) Hirschsprung disease is linked to defects in neural crest stem cell function. Science 301:972–976
Stanchina L, Baral V, Robert F, Pingault V, Lemort N, Pachnis V, Goossens M, Bondurand N (2006) Interactions between Sox10, Edn3 and Ednrb during enteric nervous system and melanocyte development. Dev Biol 295:232–249
Tam PK, Gould SJ, Martucciello G, Biddolph S, Takahashi M, Jasonni V (1996) Ret protein in the human fetal rectum. J Pediatr Surg 31:568–571
Auricchio A, Griseri P, Carpentieri ML, Betsos N, Staiano A, Tozzi A, Priolo M, Thompson H, Bocciardi R, Romeo G, Ballabio A, Ceccherini I (1999) Double heterozygosity for a RET substitution interfering with splicing and an EDNRB missense mutation in Hirschsprung disease. Am J Hum Genet 64:1216–1221
Gabriel SB, Salomon R, Pelet A, Angrist M, Amiel J, Fornage M, Attie-Bitach T, Olson JM, Hofstra R, Buys C, Steffann J, Munnich A, Lyonnet S, Chakravarti A (2002) Segregation at three loci explains familial and population risk in Hirschsprung disease. Nat Genet 31:89–93
Bolk S, Pelet A, Hofstra RM, Angrist M, Salomon R, Croaker D, Buys CH, Lyonnet S, Chakravarti A (2000) A human model for multigenic inheritance: phenotypic expression in Hirschsprung disease requires both the RET gene and a new 9q31 locus. Proc Natl Acad Sci USA 97:268–273
Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM (1996) GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell 85:1113–1124
Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, Phillips HS, Goddard A, Moore MW, Buj-Bello A, Davies AM, Asai N, Takahashi M, Vandlen R, Henderson CE, Rosenthal A (1996) Characterization of a multicomponent receptor for GDNF. Nature 382:80–83
Bar KJ, Facer P, Williams NS, Tam PK, Anand P (1997) Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprung’s disease. Gastroenterology 112:1381–1385
Pachnis V, Mankoo B, Costantini F (1993) Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119:1005–1017
Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G, Pachnis V (1999) Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development 126:2785–2797
Hofstra RM, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, Pasini B, Hoppener JW, van Amstel HK, Romeo G (1994) A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 367:375–376
Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394
Pasini B, Borrello MG, Greco A, Bongarzone I, Luo Y, Mondellini P, Alberti L, Miranda C, Arighi E, Bocciardi R (1995) Loss of function effect of RET mutations causing Hirschsprung disease. Nat Genet 10:35–40
Iwashita T, Murakami H, Asai N, Takahashi M (1996) Mechanism of ret dysfunction by Hirschsprung mutations affecting its extracellular domain. Hum Mol Genet 5:1577–1580
Pelet A, Geneste O, Edery P, Pasini A, Chappuis S, Atti T, Munnich A, Lenoir G, Lyonnet S, Billaud M (1998) Various mechanisms cause RET-mediated signaling defects in Hirschsprung’s disease. J Clin Invest 101:1415–1423
Geneste O, Bidaud C, De Vita G, Hofstra RM, Tartare-Deckert S, Buys CH, Lenoir GM, Santoro M, Billaud M (1999) Two distinct mutations of the RET receptor causing Hirschsprung’s disease impair the binding of signalling effectors to a multifunctional docking site. Hum Mol Genet 8:1989–1999
Iwashita T, Kurokawa K, Qiao S, Murakami H, Asai N, Kawai K, Hashimoto M, Watanabe T, Ichihara M, Takahashi M (2001) Functional analysis of RET with Hirschsprung mutations affecting its kinase domain. Gastroenterology 121:24–33
Kashuk CS, Stone EA, Grice EA, Portnoy ME, Green ED, Sidow A, Chakravarti A, McCallion AS (2005) Phenotype-genotype correlation in Hirschsprung disease is illuminated by comparative analysis of the RET protein sequence. Proc Natl Acad Sci USA 102:8949–8954
Edery P, Pelet A, Mulligan LM, Abel L, Attie T, Dow E, Bonneau D, David A, Flintoff W, Jan D (1994) Long segment and short segment familial Hirschsprung’s disease: variable clinical expression at the RET locus. J Med Genet 31:602–606
Yin L, Barone V, Seri M, Bolino A, Bocciardi R, Ceccherini I, Pasini B, Tocco T, Lerone M, Cywes S (1994) Heterogeneity and low detection rate of RET mutations in Hirschsprung disease. Eur J Hum Genet 2:272–280
Angrist M, Bolk S, Thiel B, Puffenberger EG, Hofstra RM, Buys CH, Cass DT, Chakravarti A (1995) Mutation analysis of the RET receptor tyrosine kinase in Hirschsprung disease. Hum Mol Genet 4:821–830
Attie T, Pelet A, Edery P, Eng C, Mulligan LM, Amiel J, Boutrand L, Beldjord C, Nihoul-Fekete C, Munnich A (1995) Diversity of RET proto-oncogene mutations in familial and sporadic Hirschsprung disease. Hum Mol Genet 4:1381–1386
Yin L, Seri M, Barone V, Tocco T, Scaranari M, Romeo G (1996) Prevalence and parental origin of de novo RET mutations in Hirschsprung’s disease. Eur J Hum Genet 4:356–358
Kusafuka T, Wang Y, Puri P (1997) Mutation analysis of the RET, the endothelin-B receptor, and the endothelin-3 genes in sporadic cases of Hirschsprung’s disease. J Pediatr Surg 32:501–504
Svensson PJ, Molander ML, Eng C, Anvret M, Nordenskjold A (1998) Low frequency of RET mutations in Hirschsprung disease in Sweden. Clin Genet 54:39–44
Inoue K, Shimotake T, Iwai N (2000) Mutational analysis of RET/GDNF/NTN genes in children with total colonic aganglionosis with small bowel involvement. Am J Med Genet 93:278–284
Hofstra RM, Wu Y, Stulp RP, Elfferich P, Osinga J, Maas SM, Siderius L, Brooks AS, vd Ende JJ, Heydendael VM, Severijnen RS, Bax KM, Meijers C, Buys CH (2000) RET and GDNF gene scanning in Hirschsprung patients using two dual denaturing gel systems. Hum Mutat 15:418–429
Sancandi M, Ceccherini I, Costa M, Fava M, Chen B, Wu Y, Hofstra R, Laurie T, Griffths M, Burge D, Tam PK (2000) Incidence of RET mutations in patients with Hirschsprung’s disease. J Pediatr Surg 35:139–142
Julies MG, Moore SW, Kotze MJ, du Plessis L (2001) Novel RET mutations in Hirschsprung’s disease patients from the diverse South African population. Eur J Hum Genet 9:419–423
Seri M, Yin L, Barone V, Bolino A, Celli I, Bocciardi R, Pasini B, Ceccherini I, Lerone M, Kristoffersson U, Larsson LT, Casasa JM, Cass DT, Abramowicz MJ, Vanderwinden JM, Kravcenkiene I, Baric I, Silengo M, Martucciello G, Romeo G (1997) Frequency of RET mutations in long- and short-segment Hirschsprung disease. Hum Mutat 9:243–249
Li JC, Ding SP, Song Y, Li MJ (2002) Mutation of RET gene in Chinese patients with Hirschsprung’s disease. World J Gastroenterol 8:1108–1111
Solari V, Ennis S, Yoneda A, Wong L, Messineo A, Hollwarth ME, Green A, Puri P (2003) Mutation analysis of the RET gene in total intestinal aganglionosis by wave DNA fragment analysis system. J Pediatr Surg 38:497–501
Gath R, Goessling A, Keller KM, Koletzko S, Coerdt W, Muntefering H, Wirth S, Hofstra RM, Mulligan L, Eng C, von Deimling A (2001) Analysis of the RET, GDNF, EDN3, and EDNRB genes in patients with intestinal neuronal dysplasia and Hirschsprung disease. Gut 48:671–675
Garcia-Barcelo M, Sham MH, Lee WS, Lui VC, Chen BL, Wong KK, Wong JS, Tam PK (2004) Highly recurrent RET mutations and novel mutations in genes of the receptor tyrosine kinase and endothelin receptor B pathways in Chinese patients with sporadic Hirschsprung disease. Clin Chem 50:93–100
Borrego S, Saez ME, Ruiz A, Gimm O, Lopez-Alonso M, Antinolo G, Eng C (1999) Specific polymorphisms in the RET proto-oncogene are over-represented in patients with Hirschsprung disease and may represent loci modifying phenotypic expression. J Med Genet 36:771–774
Fitze G, Schreiber M, Kuhlisch E, Schackert HK, Roesner D (1999) Association of RET protooncogene codon 45 polymorphism with Hirschsprung disease. Am J Hum Genet 65:1469–1473
Ceccherini I, Hofstra RM, Luo Y, Stulp RP, Barone V, Stelwagen T, Bocciardi R, Nijveen H, Bolino A, Seri M (1994) DNA polymorphisms and conditions for SSCP analysis of the 20 exons of the ret proto-oncogene. Oncogene 9:3025–3029
Garcia-Barcelo MM, Sham MH, Lui VC, Chen BL, Song YQ, Lee WS, Yung SK, Romeo G, Tam PK (2003) Chinese patients with sporadic Hirschsprung’s disease are predominantly represented by a single RET haplotype. J Med Genet 40:e122
Carrasquillo MM, McCallion AS, Puffenberger EG, Kashuk CS, Nouri N, Chakravarti A (2002) Genome-wide association study and mouse model identify interaction between RET and EDNRB pathways in Hirschsprung disease. Nat Genet 32:237–244
Fitze G, Cramer J, Ziegler A, Schierz M, Schreiber M, Kuhlisch E, Roesner D, Schackert HK (2002) Association between c135G/A genotype and RET proto-oncogene germline mutations and phenotype of Hirschsprung’s disease. Lancet 359:1200–1205
Griseri P, Pesce B, Patrone G, Osinga J, Puppo F, Sancandi M, Hofstra R, Romeo G, Ravazzolo R, Devoto M, Ceccherini I (2002) A rare haplotype of the RET proto-oncogene is a risk-modifying allele in Hirschsprung disease. Am J Hum Genet 71:969–974
Borrego S, Wright FA, Fernandez RM, Williams N, Lopez-Alonso M, Davuluri R, Antinolo G, Eng C (2003) A founding locus within the RET proto-oncogene may account for a large proportion of apparently sporadic Hirschsprung disease and a subset of cases of sporadic medullary thyroid carcinoma. Am J Hum Genet 72:88–100
Borrego S, Ruiz A, Saez ME, Gimm O, Gao X, Lopez-Alonso M, Hernandez A, Wright FA, Antinolo G, Eng C (2000) RET genotypes comprising specific haplotypes of polymorphic variants predispose to isolated Hirschsprung disease. J Med Genet 37:572–578
Fitze G, Appelt H, Konig IR, Gorgens H, Stein U, Walther W, Gossen M, Schreiber M, Ziegler A, Roesner D, Schackert HK (2003) Functional haplotypes of the RET proto-oncogene promoter are associated with Hirschsprung disease (HSCR). Hum Mol Genet 12:3207–3214
Sancandi M, Griseri P, Pesce B, Patrone G, Puppo F, Lerone M, Martucciello G, Romeo G, Ravazzolo R, Devoto M, Ceccherini I (2003) Single nucleotide polymorphic alleles in the 5′ region of the RET proto-oncogene define a risk haplotype in Hirschsprung’s disease. J Med Genet 40:714–718
Garcia-Barcelo M, Ganster RW, Lui VC, Leon TY, So MT, Lau AM, Fu M, Sham MH, Knight J, Zannini MS, Sham PC, Tam PK (2005) TTF-1 and RET promoter SNPs: regulation of RET transcription in Hirschsprung’s disease. Hum Mol Genet 14:191–204
Burzynski GM, Nolte IM, Osinga J, Ceccherini I, Twigt B, Maas S, Brooks A, Verheij J, Menacho IP, Buys CH, Hofstra RM (2004) Localizing a putative mutation as the major contributor to the development of sporadic Hirschsprung disease to the RET genomic sequence between the promoter region and exon 2. Eur J Hum Genet 12:604–612
Burzynski GM, Nolte IM, Bronda A, Bos KK, Osinga J, Plaza Menacho I, Twigt B, Maas S, Brooks AS, Verheij JB, Buys CH, Hofstra RM (2005) Identifying candidate Hirschsprung disease-associated RET variants. Am J Hum Genet 76:850–858
Emison ES, McCallion AS, Kashuk CS, Bush RT, Grice E, Lin S, Portnoy ME, Cutler DJ, Green ED, Chakravarti A (2005) A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature 434:857–863
Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A (1996) Renal and neuronal abnormalities in mice lacking GDNF. Nature 382:76–79
Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM Jr, Milbrandt J (1998) GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron 21:317–324
Shen L, Pichel JG, Mayeli T, Sariola H, Lu B, Westphal H (2002) Gdnf haploinsufficiency causes Hirschsprung-like intestinal obstruction and early-onset lethality in mice. Am J Hum Genet 70:435–447
Angrist M, Bolk S, Halushka M, Lapchak PA, Chakravarti A (1996) Germline mutations in glial cell line-derived neurotrophic factor (GDNF) and RET in a Hirschsprung disease patient. Nat Genet 14:341–344
Doray B, Salomon R, Amiel J, Pelet A, Touraine R, Billaud M, Attie T, Bachy B, Munnich A, Lyonnet S (1998) Mutation of the RET ligand, neurturin, supports multigenic inheritance in Hirschsprung disease. Hum Mol Genet 7:1449–1452
Myers SM, Salomon R, Goessling A, Pelet A, Eng C, von Deimling A, Lyonnet S, Mulligan LM (1999) Investigation of germline GFR alpha-1 mutations in Hirschsprung disease. J Med Genet 36:217–220
Salomon R, Attie T, Pelet A, Bidaud C, Eng C, Amiel J, Sarnacki S, Goulet O, Ricour C, Nihoul-Fekete C, Munnich A, Lyonnet S (1996) Germline mutations of the RET ligand GDNF are not sufficient to cause Hirschsprung disease. Nat Genet 14:345–347
Ivanchuk SM, Myers SM, Eng C, Mulligan LM (1996) De novo mutation of GDNF, ligand for the RET/GDNFR-alpha receptor complex, in Hirschsprung disease. Hum Mol Genet 5:2023–2026
Chen B, Knowles CH, Scott M, Anand P, Williams NS, Milbrandt J, Tam PK (2002) Idiopathic slow transit constipation and megacolon are not associated with neurturin mutations. Neurogastroenterol Motil 14:513–517
Eketjall S, Ibanez CF (2002) Functional characterization of mutations in the GDNF gene of patients with Hirschsprung disease. Hum Mol Genet 11:325–329
Borghini S, Bocciardi R, Bonardi G, Matera I, Santamaria G, Ravazzolo R, Ceccherini I (2002) Hirschsprung associated GDNF mutations do not prevent RET activation. Eur J Hum Genet 10:183–187
Lui VC, Samy ET, Sham MH, Mulligan LM, Tam PK (2002) Glial cell line-derived neurotrophic factor family receptors are abnormally expressed in aganglionic bowel of a subpopulation of patients with Hirschsprung’s disease. Lab Invest 82:703–712
Lang D, Epstein JA (2003) Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer. Hum Mol Genet 12:937–945
Lang D, Chen F, Milewski R, Li J, Lu MM, Epstein JA (2000) Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret. J Clin Invest 106:963–971
Zhu L, Lee HO, Jordan CS, Cantrell VA, Southard-Smith EM, Shin MK (2004) Spatiotemporal regulation of endothelin receptor-B by SOX10 in neural crest-derived enteric neuron precursors. Nat Genet 36:732–737
Cantrell VA, Owens SE, Chandler RL, Airey DC, Bradley KM, Smith JR, Southard-Smith EM (2004) Interactions between Sox10 and EdnrB modulate penetrance and severity of aganglionosis in the Sox10Dom mouse model of Hirschsprung disease. Hum Mol Genet 13(19):2289–2301
Lane PW, Liu HM (1984) Association of megacolon with a new dominant spotting gene (Dom) in the mouse. J Hered 75:435–439
Southard-Smith EM, Angrist M, Ellison JS, Agarwala R, Baxevanis AD, Chakravarti A, Pavan WJ (1999) The Sox10(Dom) mouse: modeling the genetic variation of Waardenburg-Shah (WS4) syndrome. Genome Res 9:215–225
Southard-Smith EM, Kos L, Pavan WJ (1998) Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet 18:60–64
Pingault V, Puliti A, Prehu MO, Samadi A, Bondurand N, Goossens M (1997) Human homology and candidate genes for the Dominant megacolon locus, a mouse model of Hirschsprung disease. Genomics 39:86–89
Herbarth B, Pingault V, Bondurand N, Kuhlbrodt K, Hermans-Borgmeyer I, Puliti A, Lemort N, Goossens M, Wegner M (1998) Mutation of the Sry-related Sox10 gene in dominant megacolon, a mouse model for human Hirschsprung disease. Proc Natl Acad Sci USA 95:5161–5165
Pingault V, Bondurand N, Kuhlbrodt K, Goerich DE, Prehu MO, Puliti A, Herbarth B, Hermans-Borgmeyer I, Legius E, Matthijs G, Amiel J, Lyonnet S, Ceccherini I, Romeo G, Smith JC, Read AP, Wegner M, Goossens M (1998) SOX10 mutations in patients with Waardenburg–Hirschsprung disease. Nat Genet 18:171–173
Pingault V, Guiochon-Mantel A, Bondurand N, Faure C, Lacroix C, Lyonnet S, Goossens M, Landrieu P (2000) Peripheral neuropathy with hypomyelination, chronic intestinal pseudo-obstruction and deafness: a developmental “neural crest syndrome” related to a SOX10 mutation. Ann Neurol 48:671–676
Pingault V, Girard M, Bondurand N, Dorkins H, Van Maldergem L, Mowat D, Shimotake T, Verma I, Baumann C, Goossens M (2002) SOX10 mutations in chronic intestinal pseudo-obstruction suggest a complex physiopathological mechanism. Hum Genet 111:198–206
Touraine RL, Attie-Bitach T, Manceau E, Korsch E, Sarda P, Pingault V, Encha-Razavi F, Pelet A, Auge J, Nivelon-Chevallier A, Holschneider AM, Munnes M, Doerfler W, Goossens M, Munnich A, Vekemans M, Lyonnet S (2000) Neurological phenotype in Waardenburg syndrome type 4 correlates with novel SOX10 truncating mutations and expression in developing brain. Am J Hum Genet 66:1496–1503
Inoue K, Shilo K, Boerkoel CF, Crowe C, Sawady J, Lupski JR, Agamanolis DP (2002) Congenital hypomyelinating neuropathy, central dysmyelination, and Waardenburg–Hirschsprung disease: phenotypes linked by SOX10 mutation. Ann Neurol 52:836–842
Inoue K, Tanabe Y, Lupski JR (1999) Myelin deficiencies in both the central and the peripheral nervous systems associated with a SOX10 mutation. Ann Neurol 46:313–318
Sham MH, Lui VC, Chen BL, Fu M, Tam PK (2001) Novel mutations of SOX10 suggest a dominant negative role in Waardenburg-Shah syndrome. J Med Genet 38:E30
Bondurand N, Kuhlbrodt K, Pingault V, Enderich J, Sajus M, Tommerup N, Warburg M, Hennekam RC, Read AP, Wegner M, Goossens M (1999) A molecular analysis of the Yemenite deaf-blind hypopigmentation syndrome: SOX10 dysfunction causes different neurocristopathies. Hum Mol Genet 8:1785–1789
Chan KK, Wong CK, Lui VC, Tam PK, Sham MH (2003) Analysis of SOX10 mutations identified in Waardenburg–Hirschsprung patients: differential effects on target gene regulation. J Cell Biochem 90:573–585
Dubreuil V, Hirsch MR, Pattyn A, Brunet JF, Goridis C (2000) The Phox2b transcription factor coordinately regulates neuronal cell cycle exit and identity. Development 127:5191–5201
Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF (1999) The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399:366–370
Benailly HK, Lapierre JM, Laudier B, Amiel J, Attie T, De Blois MC, Vekemans M, Romana SP (2003) PMX2B, a new candidate gene for Hirschsprung’s disease. Clin Genet 64:204–209
Garcia-Barcelo M, Sham MH, Lui VC, Chen BL, Ott J, Tam PK (2003) Association study of PHOX2B as a candidate gene for Hirschsprung’s disease. Gut 52:563–567
Miao X, Garcia-Barcelo MM, So MT, Leon TY, Lau DK, Liu TT, Chan EK, Lan LC, Wong KK, Lui VC, Tam PK (2007) Role of RET and PHOX2B gene polymorphisms in risk of Hirschsprung’s disease in Chinese population. Gut 56:736
Trochet D, Bourdeaut F, Janoueix-Lerosey I, Deville A, de Pontual L, Schleiermacher G, Coze C, Philip N, Frebourg T, Munnich A, Lyonnet S, Delattre O, Amiel J (2004) Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. Am J Hum Genet 74:761–764
Perri P, Bachetti T, Longo L, Matera I, Seri M, Tonini GP, Ceccherini I (2005) PHOX2B mutations and genetic predisposition to neuroblastoma. Oncogene 24:3050–3053
Fan J, Tam P, Woude GV, Ren Y (2004) Normalization and analysis of cDNA microarrays using within-array replications applied to neuroblastoma cell response to a cytokine. Proc Natl Acad Sci USA 101:1135–1140
Amiel J, Laudier B, ttie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S (2003) Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33:459–461
Bachetti T, Matera I, Borghini S, Di Duca M, Ravazzolo R, Ceccherini I (2005) Distinct pathogenetic mechanisms for PHOX2B associated polyalanine expansions and frameshift mutations in congenital central hypoventilation syndrome. Hum Mol Genet 14:1815–1824
Verloes A, Elmer C, Lacombe D, Heinrichs C, Rebuffat E, Demarquez JL, Moncla A, Adam E (1993) Ondine–Hirschsprung syndrome (Haddad syndrome). Further delineation in two cases and review of the literature. Eur J Pediatr 152:75–77
Lai D, Schroer B (2008) Haddad syndrome: a case of an infant with central congenital hypoventilation syndrome and Hirschsprung disease. J Child Neurol 23:341–343
Fitze G, Konig IR, Paditz E, Serra A, Schlafke M, Roesner D, Ziegler A, Schackert HK (2008) Compound effect of PHOX2B and RET gene variants in congenital central hypoventilation syndrome combined with Hirschsprung disease. Am J Med Genet A 146A:1486–1489
Lui VC, Cheng WW, Leon TY, Lau DK, Garcia-Bareclo MM, Miao XP, Kam MK, So MT, Chen Y, Wall NA, Sham MH, Tam PK (2008) Perturbation of hoxb5 signaling in vagal neural crests down-regulates ret leading to intestinal hypoganglionosis in mice. Gastroenterology 134:1104–1115
Fu M, Lui VC, Sham MH, Cheung ANY, Tam PK (2003) HOXB5 expression is spatially and temporarily regulated in human embryonic gut during neural crest cell colonization and differentiation of enteric neuroblasts. Dev Dyn 228:1–10
Garcia-Barcelo MM, Miao X, Lui VC, So MT, Ngan ES, Leon TY, Lau DK, Liu TT, Lao X, Guo W, Holden WT, Moore J, Tam PK (2007) Correlation between genetic variations in Hox clusters and Hirschsprung’s disease. Ann Hum Genet 71:526–536
Garcia-Barcelo MM, Lau DK, Ngan ES, Leon TY, Liu TT, So MT, Miao XP, Lui VC, Wong KK, Ganster RW, Cass DT, Croaker GD, Tam PK (2007) Evaluation of the thyroid transcription factor-1 gene (TITF1) as a Hirschsprung’s disease locus. Ann Hum Genet 71(Pt 6):746–754
Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M (1994) Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell 79:1267–1276
Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M (1994) Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell 79:1277–1285
Barlow A, de Graaff E, Pachnis V (2003) Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron 40:905–916
Puffenberger EG, Kauffman ER, Bolk S, Matise TC, Washington SS, Angrist M, Weissenbach J, Garver KL, Mascari M, Ladda R (1994) Identity-by-descent and association mapping of a recessive gene for Hirschsprung disease on human chromosome 13q22. Hum Mol Genet 3:1217–1225
Puffenberger EG, Hosoda K, Washington SS, Nakao K, de Wit D, Yanagisawa M, Chakravart A (1994) A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung’s disease. Cell 79:1257–1266
Attie T, Till M, Pelet A, Amiel J, Edery P, Boutrand L, Munnich A, Lyonnet S (1995) Mutation of the endothelin-receptor B gene in Waardenburg–Hirschsprung disease. Hum Mol Genet 4:2407–2409
Verheij JB, Kunze J, Osinga J, van Essen AJ, Hofstra RM (2002) ABCD syndrome is caused by a homozygous mutation in the EDNRB gene. Am J Med Genet 108:223–225
Sakai T, Nirasawa Y, Itoh Y, Wakizaka A (2000) Japanese patients with sporadic Hirschsprung: mutation analysis of the receptor tyrosine kinase proto-oncogene, endothelin-B receptor, endothelin-3, glial cell line-derived neurotrophic factor and neurturin genes: a comparison with similar studies. Eur J Pediatr 159:160–167
Amiel J, Attie T, Jan D, Pelet A, Edery P, Bidaud C, Lacombe D, Tam P, Simeoni J, Flori E, Nihoul-Fekete C, Munnich A, Lyonnet S (1996) Heterozygous endothelin receptor B (EDNRB) mutations in isolated Hirschsprung disease. Hum Mol Genet 5:355–357
Tanaka H, Moroi K, Iwai J, Takahashi H, Ohnuma N, Hori S, Takimoto M, Nishiyama M, Masaki T, Yanagisawa M, Sekiya S, Kimura S (1998) Novel mutations of the endothelin B receptor gene in patients with Hirschsprung’s disease and their characterization. J Biol Chem 273:11378–11383
Kusafuka T, Wang Y, Puri P (1996) Novel mutations of the endothelin-B receptor gene in isolated patients with Hirschsprung’s disease. Hum Mol Genet 5:347–349
Kusafuka T, Puri P (1997) Mutations of the endothelin-B receptor and endothelin-3 genes in Hirschsprung’s disease. Pediatr Surg Int 12:19–23
Auricchio A, Casari G, Staiano A, Ballabio A (1996) Endothelin-B receptor mutations in patients with isolated Hirschsprung disease from a non-inbred population. Hum Mol Genet 5:351–354
Abe Y, Sakurai T, Yamada T, Nakamura T, Yanagisawa M, Goto K (2000) Functional analysis of five endothelin-B receptor mutations found in human Hirschsprung disease patients. Biochem Biophys Res Commun 275:524–531
Fuchs S, Amiel J, Claudel S, Lyonnet S, Corvol P, Pinet F (2001) Functional characterization of three mutations of the endothelin B receptor gene in patients with Hirschsprung’s disease: evidence for selective loss of Gi coupling. Mol Med 7:115–124
Svensson PJ, Von Tell D, Molander ML, Anvret M, Nordenskjold A (1999) A heterozygous frameshift mutation in the endothelin-3 (EDN-3) gene in isolated Hirschsprung’s disease. Pediatr Res 45:714–717
Bidaud C, Salomon R, Van Camp G, Pelet A, Attie T, Eng C, Bonduelle M, Amiel J, Nihoul-Fekete C, Willems PJ, Munnich A, Lyonnet S (1997) Endothelin-3 gene mutations in isolated and syndromic Hirschsprung disease. Eur J Hum Genet 5:247–251
Edery P, Attie T, Amiel J, Pelet A, Eng C, Hofstra RM, Martelli H, Bidaud C, Munnich A, Lyonnet S (1996) Mutation of the endothelin-3 gene in the Waardenburg–Hirschsprung disease (Shah-Waardenburg syndrome). Nat Genet 12:442–444
Hofstra RM, Osinga J, Tan-Sindhunata G, Wu Y, Kamsteeg EJ, Stulp RP, Ravenswaaij-Arts C, Majoor-Krakauer D, Angrist M, Chakravarti A, Meijers C, Buys CH (1996) A homozygous mutation in the endothelin-3 gene associated with a combined Waardenburg type 2 and Hirschsprung phenotype (Shah-Waardenburg syndrome). Nat Genet 12:445–447
Yanagisawa H, Yanagisawa M, Kapur RP, Richardson JA, Williams SC, Clouthier DE, de Wit D, Emoto N, Hammer RE (1998) Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development 125:825–836
Hofstra RM, Valdenaire O, Arch E, Osinga J, Kroes H, Loffler BM, Hamosh A, Meijers C, Buys CH (1999) A loss-of-function mutation in the endothelin-converting enzyme 1 (ECE-1) associated with Hirschsprung disease, cardiac defects, and autonomic dysfunction. Am J Hum Genet 64:304–308
McCallion AS, Chakravarti A (2001) EDNRB/EDN3 and Hirschsprung disease type II. Pigment Cell Res 14:161–169
Leibl MA, Ota T, Woodward MN, Kenny SE, Lloyd DA, Vaillant CR, Edgar DH (1999) Expression of endothelin 3 by mesenchymal cells of embryonic mouse caecum. Gut 44:246–252
Sidebotham EL, Woodward MN, Kenny SE, Lloyd DA, Vaillant CR, Edgar DH (2002) Localization and endothelin-3 dependence of stem cells of the enteric nervous system in the embryonic colon. J Pediatr Surg 37:145–150
Wakamatsu N, Yamada Y, Yamada K, Ono T, Nomura N, Taniguchi H, Kitoh H, Mutoh N, Yamanaka T, Mushiake K, Kato K, Sonta S, Nagaya M (2001) Mutations in SIP1, encoding Smad interacting protein-1, cause a form of Hirschsprung disease. Nat Genet 27:369–370
Mowat DR, Croaker GD, Cass DT, Kerr BA, Chaitow J, Ades LC, Chia NL, Wilson MJ (1998) Hirschsprung disease, microcephaly, mental retardation, and characteristic facial features: delineation of a new syndrome and identification of a locus at chromosome 2q22-q23. J Med Genet 35:617–623
Garavelli L, Donadio A, Zanacca C, Banchini G, Della GE, Bertani G, Albertini G, Del Rossi C, Zweier C, Rauch A, Zollino M, Neri G (2003) Hirschsprung disease, mental retardation, characteristic facial features, and mutation in the gene ZFHX1B (SIP1): confirmation of the Mowat–Wilson syndrome. Am J Med Genet 116A:385–388
Cerruti MP, Pastore G, Zweier C, Rauch A (2004) Mowat–Wilson syndrome and mutation in the zinc finger homeo box 1B gene: a well defined clinical entity. J Med Genet 41:E16
McCallion AS, Stames E, Conlon RA, Chakravarti A (2003) Phenotype variation in two-locus mouse models of Hirschsprung disease: tissue-specific interaction between Ret and Ednrb. Proc Natl Acad Sci USA 100:1826–1831
Garcia-Barcelo MM, Fong PY, Tang CS, Miao XP, So MT, Yuan ZW, Li L, Guo WH, Liu L, Wang B, Sun XB, Huang LM, Tou JF, Wong KK, Ngan ES, Lui VC, Cherny SS, Sham PC, Tam PK (2008) Mapping of a Hirschsprung’s disease locus in 3p21. Eur J Hum Genet 16:833–840
Fernandez RM, Sanchez-Mejias A, Mena MD, Ruiz-Ferrer M, Lopez-Alonso M, Antinolo G, Borrego S (2009) A novel point variant in NTRK3, R645C, suggests a role of this gene in the pathogenesis of Hirschsprung disease. Ann Hum Genet 73:19–25
Murone M, Rosenthal A, de Sauvage FJ (1999) Hedgehog signal transduction: from flies to vertebrates. Exp Cell Res 253:25–33
Ramalho-Santos M, Melton DA, McMahon AP (2000) Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127:2763–2772
Gao B, Guo J, She C, Shu A, Yang M, Tan Z, Yang X, Guo S, Feng G, He L (2001) Mutations in IHH, encoding Indian hedgehog, cause brachydactyly type A-1. Nat Genet 28:386–388
Hellemans J, Coucke PJ, Giedion A, De Paepe A, Kramer P, Beemer F, Mortier GR (2003) Homozygous mutations in IHH cause acrocapitofemoral dysplasia, an autosomal recessive disorder with cone-shaped epiphyses in hands and hips. Am J Hum Genet 72:1040–1046
Garcia-Barcelo MM, Lee WS, Sham MH, Lui VC, Tam PK (2003) Is there a role for the IHH gene in Hirschsprung’s disease? Neurogastroenterol Motil 15:663–668
Faissner A, Kruse J, Nieke J, Schachner M (1984) Expression of neural cell adhesion molecule L1 during development, in neurological mutants and in the peripheral nervous system. Brain Res 317:69–82
Van Camp G, Vits L, Coucke P, Lyonnet S, Schrander-Stumpel C, Darby J, Holden J, Munnich A, Willems PJ (1993) A duplication in the L1CAM gene associated with X-linked hydrocephalus. Nat Genet 4:421–425
Parisi MA, Kapur RP, Neilson I, Hofstra RM, Holloway LW, Michaelis RC, Leppig KA (2002) Hydrocephalus and intestinal aganglionosis: is L1CAM a modifier gene in Hirschsprung disease? Am J Med Genet 108:51–56
Okamoto N, Wada Y, Goto M (1997) Hydrocephalus and Hirschsprung’s disease in a patient with a mutation of L1CAM. J Med Genet 34:670–671
Hofstra RM, Elfferich P, Osinga J, Verlind E, Fransen E, Lopez PJ, Die-Smulders CE, Stolte-Dijkstra I, Buys CH (2002) Hirschsprung disease and L1CAM: is the disturbed sex ratio caused by L1CAM mutations? J Med Genet 39:E11
Garcia-Barcelo MM, Tang CS, Ngan ES, Lui VC, Chen Y, So MT, Leon TY, Miao XP, Shum CK, Liu FQ, Yeung MY, Yuan ZW, Guo WH, Liu L, Sun XB, Huang LM, Tou JF, Song YQ, Chan D, Cheung KM, Wong KK, Cherny SS, Sham PC, Tam PK (2009) Genome-wide association study identifies NRG1 as a susceptibility locus for Hirschsprung’s disease. Proc Natl Acad Sci USA 106:2694–2699
Badner JA, Sieber WK, Garver KL, Chakravarti A (1990) A genetic study of Hirschsprung disease. Am J Hum Genet 46:568–580
Borst MJ, VanCamp JM, Peacock ML, Decker RA (1995) Mutational analysis of multiple endocrine neoplasia type 2A associated with Hirschsprung’s disease. Surgery 117:386–391
Mulligan LM, Eng C, Attie T, Lyonnet S, Marsh DJ, Hyland VJ, Robinson BG, Frilling A, Verellen-Dumoulin C, Safar A (1994) Diverse phenotypes associated with exon 10 mutations of the RET proto-oncogene. Hum Mol Genet 3:2163–2167
Reynolds LF, Eng C (1995) RET mutations in multiple endocrine neoplasia type 2 and Hirschsprung disease. Curr Opin Pediatr 7:702–709
Cosma MP, Panariello L, Quadro L, Dathan NA, Fattoruso O, Colantuoni V (1996) A mutation in the RET proto-oncogene in Hirschsprung’s disease affects the tyrosine kinase activity associated with multiple endocrine neoplasia type 2A and 2B. Biochem J 314(Pt 2):397–400
Inoue K, Shimotake T, Inoue K, Tokiwa K, Iwai N (1999) Mutational analysis of the RET proto-oncogene in a kindred with multiple endocrine neoplasia type 2A and Hirschsprung’s disease. J Pediatr Surg 34:1552–1554
Romeo G, Ceccherini I, Celli J, Priolo M, Betsos N, Bonardi G, Seri M, Yin L, Lerone M, Jasonni V, Martucciello G (1998) Association of multiple endocrine neoplasia type 2 and Hirschsprung disease. J Intern Med 243:515–520
Sijmons RH, Hofstra RM, Wijburg FA, Links TP, Zwierstra RP, Vermey A, Aronson DC, Tan-Sindhunata G, Brouwers-Smalbraak GJ, Maas SM, Buys CH (1998) Oncological implications of RET gene mutations in Hirschsprung’s disease. Gut 43:542–547
Bodmer W, Bonilla C (2008) Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet 40:695–701
Altshuler D, Daly MJ, Lander ES (2008) Genetic mapping in human disease. Science 322:881–888
Fu M, Lui VC, Sham MH, Pachnis V, Tam PK (2004) Sonic Hedgehog regulates the proliferation, differentiation and migration of enteric neural crest cells in gut. J Cell Biol 166(5):673–684
Ngan ES, Lee KY, Sit FY, Poon HC, Chan JK, Sham MH, Lui VC, Tam PK (2007) Prokineticin-1 modulates proliferation and differentiation of enteric neural crest cells. Biochim Biophys Acta 1773(4):536–545
Acknowledgments
The authors would like to acknowledge the research grants HKU 765407M and HKU 775907M from the Hong Kong Research Grants Council to MGB and PT, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tam, P.K.H., Garcia-Barceló, M. Genetic basis of Hirschsprung’s disease. Pediatr Surg Int 25, 543–558 (2009). https://doi.org/10.1007/s00383-009-2402-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-009-2402-2