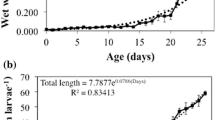
Abstract
Decapod crustaceans grow discontinuously and gain size through complex molt processes. The molt comprises the loss of the old cuticle and, moreover, substantial reduction and re-organization of muscles and connective tissues. In adult lobsters, the muscle tissue of the massive claws undergoes significant atrophy of 40–75% before ecdysis. The degradation of this tissue is facilitated by calcium-dependent proteases and by the proteasome, an intra-cellular proteolytic multi-enzyme complex. In contrast to the adults, the involvement of the proteasome during the larval development is yet not validated. Therefore, we developed micro-methods to measure the 20S and the 26S proteasomal activities within mg- and sub-mg-quantities of the larval claw tissue of the European lobster, Homarus gammarus. Within the three larval stages (Z1–3) we distinguished between sub-stages of freshly molted/hatched (post-molt), inter-molt, and ready to molt (pre-molt) larvae. Juveniles were analyzed in the post-molt and in the inter-molt stage. The trypsin-like, the chymotrypsin-like, and the peptidyl-glutamyl peptide hydrolase activity (PGPH) of the 20S proteasome increased distinctly from freshly hatched larvae to pre-molt Z1. During the Z2 stage, the activities were highest in the post-molt animals, decreased in the inter-molt animals and increased again in the pre-molt animals. A similar but less distinct trend was evident in the Z3 stages. In the juveniles, the proteasomal activities decreased toward the lowest values. A similar pattern was present for the chymotrypsin-like activity of the 26S proteasome. The results show that the proteasome plays a significant role during the larval development of lobsters. This is not only reflected by the elevated activities, but also by the continuous change of the trypsin/chymotrypsin-ratio which may indicate a shift in the subunit composition of the proteasome and, thus, a biochemical adjustment to better cope with elevated protein turnover rates during larval development.








Similar content being viewed by others
References
Ádám G, Gausz J, Noselli S, Kurucz E, Andó I, Udvardy A (2004) Tissue and developmental stage-dependent changes in the subcellular localization of the 26S proteasome in the ovary of Drosophila melanogaster. Gene Expr Patterns 4:329–333
Ahn JY, Hong SO, Kwak KB, Kang SS, Tanaka K, Ichihara A, Ha DB, Chung CH (1991) Developmental regulation of proteolytic activities and subunit pattern of 20S proteasome in chick embryonic muscle. J Biol Chem 266:15746–15749
Asher G, Reuven N, Shaul Y (2006) 20S proteasome and protein degradation “by default”. BioEssays 28:844–849
Baumeister W, Walz J, Zühl F, Seemüller E (1998) The proteasome: paradigm of a self-compartmentalizing protease. Cell 92:367–380
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–256
Cardozo C, Eleuteri AM, Orlowski M (1995) Differences in catalytic activities and subunit pattern of multicatalytic proteinase complexes (proteasomes) isolated from bovine pituitary, lung and liver. J Biol Chem 270:22645–22651
Charmantier G, Charmantier-Daures M, Aiken DE (1991) Metamorphosis in the lobster Homarus (Decapoda): a review. J Crust Biol 11:481–495
Coux O, Tanaka K, Goldberg AL (1996) Structure and function of the 20S and the 26S proteasomes. Annu Rev Biochem 65:801–847
Covi JA, Bader BD, Chang ES, Mykles DL (2010) Molt cycle regulation of protein synthesis in skeletal muscle of the blackback land crab, Gecarcinus lateralis, and the differential expression of a myostatin-like factor during atrophy induced by molting or unweighting. J Exp Biol 213:172–183
Dahlmann B, Ruppert T, Kuehn LO, Merforth S, Kloetzel PM (2000) Different proteasome subtypes in a single tissue exhibit different enzymatic properties. J Mol Biol 303:643–653
Davies KJA (2001) Degradation of oxidized proteins by the 20S proteasome. Biochimie 83:301–310
De Diego JL, Katz JM, Marshall P, Gutierrez B, Manning JE, Nussenzweig V, Gondalez J (2001) The ubiquitin-proteasome pathway plays an essential role in proteolysis during Trypanosoma cruzi remodeling. Biochemistry 40:1053–1062
De Mot R, Nagy I, Walz J, Baumeister W (1999) Proteasomes and other self-compartmentalizing proteases in prokaryotes. Trends Microbiol 7:88–92
Drews O, Wildgruber R, Zong C, Sukop U, Nissum M, Weber G, Gomes AV, Ping P (2007) Mammalian proteasome subpopulations with distinct molecular compositions and proteolytic activities. Mol Cell Proteomics 6:2021–2031
El Haj A, Clarke S, Harrison P, Chang E (1996) In vivo muscle protein synthesis rates in the American lobster Homarus americanus during the molt cycle and in response to 20-hydroxyecdysone. J Exp Biol 199:579–585
Frisan F, Levitsky V, Polack A, Masucci MG (1998) Phenotype-dependent differences in proteasome subunit composition and cleavage specificity in B cell lines. J Immunol 160:3281–3289
Galastegui N, Groll M (2010) The 26S proteasome: assembly and function of a destructive machine. Trends Biochem Sci 35:634–642
Gomes AV, Young GW, Wang Y, Zong C, Eghbali M, Drews O, Lu H, Stefani E, Ping P (2009) Contrasting proteome biology and functional heterogeneity of the 20S proteasome complexes in mammalian tissues. Mol Cell Proteomics 8:302–315
Götze S, Saborowski R (2011) NanoDrop fluorometry adopted for microassays of proteasomal enzyme activities. Anal Biochem 413:203–205. doi:10.1016/j.ab.2011.02.023
Grune T, Merker K, Sandig G, Davies KJA (2003) Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Comm 305:709–718
Haass C, Kloetzel PM (1989) The drosophila proteasome undergoes changes in its subunit pattern during development. Exp Cell Res 180:243–252
Hochstrasser M (1995) Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Op Cell Biol 7:215–223
Hong SO, Ahn JY, Lee CS, Kang MS, Ha DB, Tanaka K, Chung CH (1994) Tissue-specific expression of the subunits of chick 20S proteasomes. Biochem Mol Biol Int 32:723–729
Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA (2003) Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys 421:67–76
Ismail SZM, Mykles DL (1992) Differential molt-induced atrophy in the dimorphic claws of the male fiddler crabs, Uca pugnax. J Exp Zool 263:18–31
Jariel-Encontre I, Bossis G, Piechaczyk M (2008) Ubiquitin independent degradation of proteins by the proteasome. Biochem Biophys Acta Rec Cancer 1786:153–177
Kisselev AF, Goldberg AL (2001) Proteasome inhibitors: from research tools to drug candidates. Chem Biol 8:739–758
Kisselev AF, Akopian TN, Castillo V, Goldberg AL (1999) Proteasome active sites allosterically regulate each other, suggesting a cyclical bite–chew mechanism for protein breakdown. Mol Cell 4:395–402
Kisselev AF, Kaganowich D, Goldberg AL (2002) Binding of hydrophobic peptides to several non-catalytic sites promotes peptide hydrolysis by all active sites of 20S proteasomes. Evidence for peptide-induced channel opening in the α-rings. J Biol Chem 277:22260–22270
Kisselev AF, Garcia-Calvo M, Overkleeeft HS, Peterson E, Pennington MW, Ploegh HL, Thornberry NA, Goldberg AL (2003) The caspase-like sites of proteasomes, their substrate specificity, new inhibitors and substrates, and allosteric interactions with the trypsin-like sites. J Biol Chem 278:35869–35877
Kisselev A, Callard A, Goldberg AL (2006) Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem 281:8582–8590
Klein U, Gernold M, Kloetzel P-M (1990) Cell-specific accumulation of Drosophila proteasome (MCP) during early development. Journal Cell Biol 111:2275–2282
Koenders A, Yu X, Chang ES, Mykles DL (2002) Ubiquitin and actin expression in claw muscles of land crab, Gecarcinus lateralis, and American lobster, Homarus americanus: differential expression of ubiquitin in two slow muscle fiber types during molt-induced atrophy. J Exp Zool 292:618–632
Liu C-W, Corboy MJ, DeMartino GN, Thomas PJ (2003) Endoproteolytic activity of the proteasome. Science 299:408–411
Meng L, Mohan R, Kwork BHB, Elofsson M, Sin N, Crews CM (1999) Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo anti-inflammatory activity. Proc Natl Acad Sci USA 96:10403–10408
Mykles DL (1990) Calcium-dependent proteolysis in crustacean claw closer muscle maintained in vitro. J Exp Zool 256:16–30
Mykles DL (1996) Differential effects of bovine PA28 on six peptidase activities of the lobster muscle proteasome (MCP). Arch Biochem Biophys 325:77–81
Mykles DL (1997) Biochemical properties of insect and crustacean proteasomes. Mol Biol Rep 24:133–138
Mykles DL (1998) Intracellular proteinases of invertebrates: calcium-dependent and proteasome/ubiquitin-dependent systems. Int Rev Cytol 184:157–289
Mykles DL (1999a) Proteolytic processes underlying molt-induced claw muscle atrophy in decapod crustaceans. Am Zool 39:541–551
Mykles DL (1999b) Structure and functions of arthropod proteasomes. Mol Biol Rep 26:103–111
Mykles DL, Haire MF (1991) Sodium dodecyl sulfate and heat induce two distinct forms of lobster muscle multicatalytic proteinase: the heat-activated form degrades myofibrillar proteins. Arch Biochem Biophys 288:543–551
Mykles DL, Skinner DM (1981) Preferential loss of thin filaments during molt-induced atrophy in crab claw muscle. J Ultrastruc Res 75:314–325
Naujokat C, Hoffmann S (2002) Role and function of the 26S proteasome in proliferation and apoptosis. Lab Invest 82:965–980
Orlowski M, Wilk S (2003) Ubiquitin-independent proteolytic function of the proteasome. Arch Biochem Biophys 415:1–5
Pelletier S, Schuurman KG, Berkers CR, Ovaa H, Heck AJR, Raijmakers R (2010) Quantifying cross-tissue diversity in proteasome complexes by mass spectrometry. Mol BioSyst 6:1450–1453
Richter-Ruoff B, Wolf DH (1993) Proteasome and cell cycle: evidence for a regulatory role of the proteasome on mitotic cyclins in yeast. FEBS Lett 336:34–36
Schmalenbach I, Fanke HD (2010) Potential impact of climate warming on the recruitment of an economically and ecologically species, the European lobster (Homarus gammarus) at Helgoland, North Sea. Mar Biol 157:1127–1135
Shean BS, Mykles DL (1995) Polyubiquitin in crustacean striated muscle: increased expression and conjugation during molt-induced claw muscle atrophy. Biochim Biophys Acta 1264:312–322
Skinner DM (1966) Macromolecular changes associated with the growth of crustacean tissues. Am Zool 6:235–242
Verras M, Gourzi P, Kalosaka K, Zacharopoulou A, Mintzas AC (2008) cDNA cloning, characterization, and developmental expression of the 20S proteasome α5 subunit in the Mediterranean fruit fly Ceratitis capitata. Arch Insect Biochem Physiol 67:120–129
Voges D, Zwickel P, Baumeister W (1999) The 26S proteasome: a molecular machine designed for controlled proteolysis. An Rev Biochem 68:1015–1068
Acknowledgments
We are grateful to Dr. Isabel Schmalenbach from the Marine Station Helgoland for providing the lobster larvae and to Kristine Reuter for laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Götze, S., Saborowski, R. Proteasomal activities in the claw muscle tissue of European lobster, Homarus gammarus, during larval development. J Comp Physiol B 181, 861–871 (2011). https://doi.org/10.1007/s00360-011-0574-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0574-2