Abstract
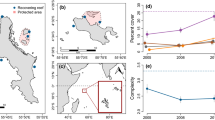
Predicting the response of coral reefs to large-scale mortality induced by climate change will depend greatly on the factors that influence recovery after bleaching events. We experimentally transplanted hard corals from a shallow reef with highly variable seawater temperature (23–36°C) to three unfished marine parks and three fished reefs with variable coral predator abundance and benthic cover. The transplanted corals were fragmented colonies collected from a reef that was relatively undisturbed by the 1997–1998 warm-water temperature anomaly, one of the most extreme thermal events of the past century, and it was assumed that they would represent corals likely to succeed in the future temperature environment. We examined the effects of four taxa, two fragment sizes, an acclimation period, benthic cover components, predators and tourists on the survival of the coral fragments. We found the lowest survival of transplants occurred in the unfished marine parks and this could be attributed to predation and not tourist damage. The density of small coral recruits approximately 6 months after the spawning season was generally moderate (~40–60/m2), and not different on fished and unfished reefs. Coral recovery between 1998 and 2002 was variable (0–25%), low (mean of 6.5%), and not different between fished and unfished reefs. There was high variability in coral mortality among the three unfished areas despite low variation in estimates of predator biomass, with the highest predation occurring in the Malindi MNP, a site with high coralline algal cover. Stepwise multiple regression analysis with 14 variables of coral predators and substratum showed that coralline algae was positively, and turf algae negatively associated with mortality of the transplants, with all other variables being statistically insignificant. This suggests that alternate food resources and predator choices are more important than predator biomass in determining coral survival. Nonetheless, large predatory fish in areas dominated by coralline algae may considerably retard recovery of eurythermal corals. This will not necessarily retard total hard coral recovery, as other more predator-tolerant taxa can recover. Based on the results, global climate change will not necessarily favor eurythermal over stenothermal coral taxa in remote or unfished reefs, where predation is a major cause of coral mortality.






Similar content being viewed by others
References
Baker AC (2001) Reef corals bleach to survive change. Nature 411:765–766
Berkelmans R, Willis BL (1999) Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18:219–228
Bradbury R, Seymour R (1997) Waiting for COTS. In: Proceedings of the 8th international coral reef symposium, vol 2, pp 1357–1362
Bradbury RH, Hammond LS, Moran PJ, Reichelt RE (1985) Coral reef communities and the crown-of-thorns starfish: evidence for qualitatively stable cycles. J Theoret Biol 113:69–80
Brown BE, Dunne RP, Goodson MS, Douglas AE (2002) Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs 21:119–126
Bruckner AW, Bruckner RJ (1998) Destruction of coral by Sparisoma viride. Coral Reefs 17:350
Bruckner AW, Bruckner RJ, Sollins P (2000) Parrotfish predation on live coral: “spot biting” and “focused biting”. Coral Reefs 19:50
Bruggemann JH, Kuyper WM, Breeman AM (1994b) Comparative analysis of foraging and habitat use by the sympatric Caribbean parrotfish Scarus vetula and Sparisoma viride (Scaridae). Mar Ecol Prog Ser 112:51–66
Bruggemann JH, Madelein JHVO, Breeman AM (1994a) Foraging by the stoplight parrotfish Sparisoma viride. I. Food selection in different, socially determined habitats. Mar Ecol Prog Ser 106:41–55
Carpenter RC (1981) Grazing by Diadema antillarum (Philippi) and its effects on the benthic algal community. J Mar Res 39:749–765
Charles CD, Hunter DE, Fairbanks RD (1997) Interaction between the ENSO and the Asian Monsoon in a coral record of tropical climate. Science 277:925–928
Choat JH (1991) The biology of herbivorous fishes on coral reefs. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic, New York, pp 120–155
Clark S, Edwards AJ (1995) Coral transplantation as an aid to reef rehabilitation: evaluation of a case study in the Maldive Islands. Coral Reefs 14:201–213
Coles SL (1997) Reef corals occurring in a highly fluctuating temperature environment at Fahal Island, Gulf of Oman (Indian Ocean). Coral Reefs 16:269–272
Coles SL, Brown BE (2003) Coral bleaching—capacity for acclimatization and adaptation. Adv Mar Biol 46:183–223
Connell JH (1997) Disturbance and recovery of coral assemblages. Coral Reefs 16:S101-S113
Cox E (1984) Resource use by corallivorous butterflyfishes (family Chaetodontidae) in Hawaii. Bull Mar Sci 54:535–545
Cumming RL (1999) Predation on reef-building corals: multiscale variation in the density of three corallivorous gastropods, Drupella spp. Coral Reefs 18:147–157
Done TJ (1987) Simulation of the effects of Acanthaster planci on the population structure of massive corals in the genus Porites: evidence of population resilience? Coral Reefs 6:75–90
Done TJ (1988) Simulation of recovery of pre-disturbance size structure in populations of Porites spp. damaged by the crown of thorns starfish Acanthaster planci. Mar Biol 100:51–61
Done T (1992) Constancy and change in some Great Barrier Reef coral communities: 1980–1990. Amer Zool 32:665–662
Findley JS, Findley MT (2001) Global, regional, and local patterns in species richness and abundance of butterflyfishes. Ecol Monogr 71:69–91
Fong P, Glynn PW (1998) A dynamic size-structured population model: does disturbance control size structure of a population of the massive coral Gardineroseris planulata in the Eastern Pacific? Mar Biol 130:663–674
Fong P, Glynn PW (2000) A regional model to predict coral population dynamics in response to EI Niño-Southern Oscillation. Ecol Appl 10:842–854
Gleason DF, Wellington GM (1993) Ultraviolet radiation and coral bleaching. Nature 365:836–838
Glynn PW (2000) El Nino-Southern Oscillation mass mortalities of reef corals: a model of high temperature marine extinctions?. In: Insalaco E, Skelton PW, Palmers TJ (eds) Carbonate platform systems: components and interactions. Geological Society of London, London, pp 117–133
Gochfeld DJ (2004) Predation-induced morphological and behavioral defenses in a hard coral: implications for foraging behaviour of coral-feeding butterflyfishes. Mar Ecol Prog Ser 267:145–158
Goreau T, McClanahan T, Hayes R, Strong A (2000) Conservation of coral reefs after the 1998 global bleaching event. Conserv Biol 14:5–15
Harmelin-Vivien ML, Bouchon-Navaro Y (1983) Feeding diets and significance of coral feeding among chaetodontid fishes in Moorea (French Polynesia). Coral Reefs 2:119–127
Hawkins JP, Roberts CM (1993) Effects of recreational scuba diving on coral reefs: trawling on reef-flat communities. J Appl Ecol 30:25–30
Heyward AJ, Negri AP (1999) Natural inducers for coral larval metamorphosis. Coral Reefs 18:273–279
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866
Hoegh-Guldberg O, Salvat B (1995) Periodic mass-bleaching and elevated sea temperatures: bleaching of outer reef slope communities in Moorea, French Polynesia. Mar Ecol Prog Ser 121:181–190
Hughes TP, Tanner JE (2000) Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 81:2250–2263
Huppert A, Stone L (1998) Chaos in the Pacific’s coral bleaching cycle. Amer Nat 152:447–459
Jokiel PL, Coles SL (1977) Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar Biol 43:201–208
Kleypas JA, Buddemeier RW, Archer D, Gattuso JP, Langdon C, Opdyke BN (1999) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284:118–120
Knowlton N, Lang JC, Keller BD (1988) Fates of staghorn coral fragments on hurricane-damaged reefs in Jamaica: the role of predators. In: Proceedings of the 6th international coral reef symposium, vol 1, pp 83–88
Lieske E, Myers R (1994) Coral reef fishes: Indo-Pacific and Caribbean. Harper Collins, London
Littler MM, Taylor PR, Littler DS (1989) Complex interactions in the control of coral zonation on a Caribbean reef flat. Oecologia 80:331–340
Marcus J, Thorhaug A (1981) Pacific versus Atlantic responses of the subtropical hermatypic coral Porites spp. to temperature and salinity effects. Proc 4th Int Coral Reef Symp 2:15–20
McClanahan TR (1994a) Kenyan coral reef lagoon fish: effects of fishing, substrate complexity, and sea urchins. Coral Reefs 13:231–241
McClanahan TR (1994b) Coral-eating snail Drupella cornus population increases in Kenyan coral reef lagoons. Mar Ecol Prog Ser 115:131–137
McClanahan TR (1997) Primary succession of coral-reef algae: differing patterns on fished versus unfished reefs. J Exp Mar Biol Ecol 218:77–102
McClanahan TR (1998) Predation and the distribution and abundance of tropical sea urchin populations. J of Exp Mar Biol Ecol 221:231–255
McClanahan TR (2000) Bleaching damage and recovery potential of Maldivian coral reefs. Mar Poll Bull 40:587–597
McClanahan TR (2004) The relationship between bleaching and mortality of common corals. Mar Biol 144:1239–1245
McClanahan TR, Arthur R (2001) The effect of marine reserves and habitat on populations of East African coral reef fishes. Ecol Appl 11:559–569
McClanahan TR, Kaunda-Arara B (1996) Fishery recovery in a coral-reef marine park and its effect on the adjacent fishery. Conserv Biol 10:1187–1199
McClanahan TR, Maina J (2003) Response of coral assemblages to the interaction between natural temperature variation and rare warm-water events. Ecosystems 6:551–563
McClanahan TR, Mutere JC (1994) Coral and sea urchin assemblage structure and interrelationships in kenyan reef lagoons. Hydrobiologia 286:109–124
McClanahan TR, Muthiga NA, Mangi S (2001) Coral and algal response to the 1998 coral bleaching and mortality: interaction with reef management and herbivores on Kenyan reefs. Coral Reefs 19:380–391
McClanahan TR, Maina J, Pet-Soede L (2002) Effects of the 1998 coral mortality event on Kenyan coral reefs and fisheries. Ambio 31:543–550
McClanahan TR, Baird AH, Marshall PA, Toscano MA (2004) Comparing bleaching and mortality responses of hard corals between southern Kenya and the Great Barrier Reef, Australia. Mar Poll Bull 48:327–335
Miller MW, Hay ME (1998) Effects of fish predation and seaweed competition on the survival and growth of corals. Oecologia 113:231–238
Muthiga NA, McClanahan TR (1997) The effect of visitor use on the hard coral communities of the Kisite Marine Park, Kenya. In: Proceedings of the 8th international coral reef symposium, vol 2, pp 1879–1882
Nakamura T, van Woesik R (2001) Differential survival of corals during the 1998 bleaching event is partially explained by water-flow rates and passive diffusion. Mar Ecol Prog Ser 212:301–304
Neudecker S (1977) Transplant experiments to test the effect of grazing on coral distribution. In: Proceedings of the 3rd international coral reef symposium, vol 1, pp 317–323
Neudecker S (1979) Effects of grazing and browsing fishes on zonation of corals in Guam. Ecology 60:666–672
Pearson RG (1981) Recovery and recolonization of coral reefs. Mar Ecol Prog Ser 4:105–122
Randall JE (1974) The effect of fishes on coral reefs. In: Proceedings of the 2nd international coral reef symposium, vol 1, pp 159–166
Rice WR (1989) Analyzing tables of statistical test. Evolution 43:223–225
Rowan R, Knowlton N (1995) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Proc Natl Acad Sci USA 92:2850–2853
Saji NH, Goswami BN, Vinayachandran PN, Yamagata T (1999) A dipole mode in the tropical Indian Ocean. Nature 401:360–363
Sall J, Lehman A (1996) JMP start statistics. Duxbury, Belmont
Sammarco PW (1980) Diadema and its relationship to coral spat mortality: grazing, competition, and biological disturbance. J Exp Mar Biol Ecol 45:245–272
Sheppard CRC (2003) Predicted recurrences of mass coral mortality in the Indian Ocean. Nature 425:294–297
Urban FE, Cole JE, Overpeck JT (2000) Influence of mean climate change on climate variability from a 155-year tropical Pacific coral record. Nature 407:989–990
Veron J (2000) Corals of the world, 1st edn. Australian Institute of Marine Science, Townsville
Ware JR (1997) The effect of global warming on coral reefs: acclimate or die. In: Proceedings of the 8th international coral reef symposium, vol 1, pp 527–532
Acknowledgments
This study received support from The Wildlife Conservation Society with partial financial support from the Dutch Wetland’s Program. The assistance of Kenya Wildlife Service in the three marine parks is greatly appreciated. Kenya’s Office of the President provided permission to undertake research. C. Starger received support from the Center for Environmental Research and Conservation at Columbia University, P. Herron-Perez from the Department of Marine Sciences and Coastal Management at the University of New-castle upon Tyne and E. Dusek from the Department of Biological Sciences at Stanford University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Environmental Editor Bruce Hatcher
Rights and permissions
About this article
Cite this article
McClanahan, T.R., Maina, J., Starger, C.J. et al. Detriments to post-bleaching recovery of corals. Coral Reefs 24, 230–246 (2005). https://doi.org/10.1007/s00338-004-0471-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-004-0471-1