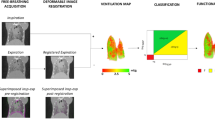
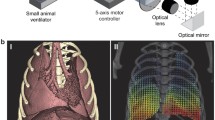
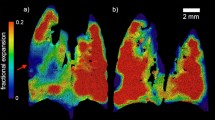
Abstract
Objectives
To evaluate the feasibility of using automatic quantitative analysis of breath hold gated micro-CT images to detect and monitor disease in a mouse model of chronic pulmonary inflammation, and to compare image-based measurements with pulmonary function tests and histomorphometry.
Material and methods
Forty-nine A/J mice were used, divided into control and inflammation groups. Chronic inflammation was induced by silica aspiration. Fourteen animals were imaged at baseline, and 4, 14, and 34 weeks after silica aspiration, using micro-CT synchronized with ventilator-induced breath holds. Lung input impedance was measured as well using forced oscillation techniques. Five additional animals from each group were killed after micro-CT for comparison with histomorphometry.
Results
At all time points, micro-CT measurements show statistically significant differences between the two groups, while first differences in functional test parameters appear at 14 weeks. Micro-CT measurements correlate well with histomorphometry and discriminate diseased and healthy groups better than functional tests.
Conclusion
Longitudinal studies using breath hold gated micro-CT are feasible on the silica-induced model of chronic pulmonary inflammation, and automatic measurements from micro-CT images correlate well with histomorphometry, being more sensitive than functional tests to detect lung damage in this model.








Similar content being viewed by others
Abbreviations
- AUC:
-
area under the ROC curve
- C:
-
compliance
- DAF:
-
damaged area fraction
- DLVF:
-
damaged lung volume fraction
- G:
-
tissue damping
- H:
-
elastance
- I:
-
inertance
- micro-CT:
-
micro-computed X-ray tomography
- MLVI:
-
mean lung volume intensity
- R:
-
resistance
- RRB:
-
radius of right mainstem bronchus
- RLB:
-
radius of left mainstem bronchus
- ROC:
-
receiver operating characteristic
- TLC:
-
total lung capacity
References
Shapiro SD (2000) Animal models for COPD. Chest 117:223S–227S
Thrall RS, McCormick JR, Jack RM, McReynolds RA, Ward PA (1979) Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol 95:117–130
Tuveson DA, Jacks T (1999) Modeling human lung cancer in mice: similarities and shortcomings. Oncogene 18:5318–5324. doi:10.1038/sj.onc.1203107
Zosky GR, Sly PD (2007) Animal models of asthma. Clin Exp Allergy 37:973–988. doi:10.1111/j.1365-2222.2007.02740.x
Suzuki N, Ohta K, Horiuchi T, Takizawa H, Ueda T, Kuwabara M, Shiga J, Ito K (1996) T lymphocytes and silica-induced pulmonary inflammation and fibrosis in mice. Thorax 51:1036–1042
Johnson KA (2007) Imaging techniques for small animal imaging models of pulmonary disease: micro-CT. Toxicol Pathol 35:59–64. doi:10.1080/01926230601184262
Froese AR, Ask K, Labiris R, Farncombe T, Warburton D, Inman MD, Gauldie J, Kolb M (2007) Three-dimensional computed tomography imaging in an animal model of emphysema. Eur Respir J 30:1082–1089. doi:10.1183/09031936.00000507
De Clerck NM, Meurrens K, Weiler H, Van Dyck D, Van Houtte G, Terpstra P, Postnov AA (2004) High-resolution X-ray microtomography for the detection of lung tumors in living mice. Neoplasia 6:374–379
Cavanaugh D, Travis EL, Price RE, Gladish G, White RA, Wang M, Cody DD (2006) Quantification of bleomycin-induced murine lung damage in vivo with micro-computed tomography. Acad Radiol 13:1505–1512. doi:10.1016/j.acra.2006.08.011
Ask K, Labiris R, Farkas L, Moeller A, Froese A, Farncombe T, McClelland GB, Inman M, Gauldie J, Kolb MR (2008) Comparison between conventional and “clinical” assessment of experimental lung fibrosis. J Transl Med 6:16. doi:10.1186/1479-5876-6-16
Ford NL, Nikolov HN, Norley CJ, Thornton MM, Foster PJ, Drangova M, Holdsworth DW (2005) Prospective respiratory-gated micro-CT of free breathing rodents. Med Phys 32:2888–2898
Cody DD, Nelson CL, Bradley WM, Wislez M, Juroske D, Price RE, Zhou X, Bekele BN, Kurie JM (2005) Murine lung tumor measurement using respiratory-gated micro-computed tomography. Invest Radiol 40:263–269
Ford NL, Wheatley AR, Holdsworth DW, Drangova M (2007) Optimization of a retrospective technique for respiratory-gated high speed micro-CT of free-breathing rodents. Phys Med Biol 52:5749–5769. doi:10.1088/0031-9155/52/19/002
Namati E, Chon D, Thiesse J, Hoffman EA, Jd R, Ross A, McLennan G (2006) In vivo micro-CT lung imaging via a computer-controlled intermittent iso-pressure breath hold (IIBH) technique. Phys Med Biol 51:6061–6075
Jobse BN, Johnson JR, Farncombe TH, Labiris R, Walker TD, Goncharova S, Jordana M (2009) Evaluation of allergic lung inflammation by computed tomography in a rat model in vivo. Eur Respir J 33:1437–1447. doi:10.1183/09031936.00087508
Lee HJ, Goo JM, Kim NR, Kim MA, Chung DH, Son KR, Kim HC, Lee CH, Park CM, Chun EJ, Im JG (2008) Semiquantitative measurement of murine bleomycin-induced lung fibrosis in in vivo and postmortem conditions using microcomputed tomography: Correlation with pathologic scores–initial results. Invest Radiol 43:453–460. doi:10.1097/RLI.0b013e31816900ec
Irvin CG, Bates JH (2003) Measuring the lung function in the mouse: the challenge of size. Respir Res 4:4
Bates JH, Irvin CG (2003) Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol 94:1297–1306. doi:10.1152/japplphysiol.00706.2002
Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ (1992) Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72:168–178
Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ (2006) Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res 32:181–199. doi:10.1080/01902140600817465
Rivera B, Miller S, Brown E, Price R (2005) A novel method for endotracheal intubation of mice and rats used in imaging studies. Contemp Top Lab Anim Sci 44:52–55
Allen GB, Suratt BT, Rinaldi L, Petty JM, Bates JH (2006) Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291:L710–717. doi:10.1152/ajplung.00532.2005
Artaechevarria X, Pérez-Martín D, Reinhardt JM, Muñoz-Barrutia A, Ortiz de Solorzano C (2009) Automated quantitative analysis of a mouse model of chronic pulmonary inflammation using micro X-ray computed tomography. MICCAI. Available via http://www.lungworkshop.org/2009/proc2009/115.pdf. Accessed 20 April 2010
Hu S, Hoffman EA, Reinhardt JM (2001) Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans Med Imaging 20:490–498. doi:10.1109/42.929615
Artaechevarria X, Perez-Martín D, Ceresa M, de Biurrun G, Blanco D, Montuenga LM, van Ginneken B, Ortiz-de-Solorzano C, Munoz-Barrutia A (2009) Airway segmentation and analysis for the study of mouse models of lung disease using micro-CT. Phys Med Biol 54:7009–7024
Fawcett T (2006) An introduction to ROC analysis. Pattern Recogn Lett 27:861–874
Eaton JW, Bateman D, Hauberg S (2002) GNU Octave manual. Network Theory Limited
Saffiotti U, Williams A, Daniel L, Kaighn M, Mao Y, Shi X (1996) Carcinogenesis by crystalline silica: animal, cellular and molecular studies. In: Castranova V, Vallyathan V, Wallace WE (eds) Silica and silica-induced lung diseases, 1st edn. CRC, Boca Raton, pp 345–381
Ford NL, Thornton MM, Holdsworth DW (2003) Fundamental image quality limits for microcomputed tomography in small animals. Med Phys 30:2869–2877
Hu S, Hoffman EA, Reinhardt JM (2001) Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans Med Imaging 20:490–498
Gietema HA, Schilham AM, van Ginneken B, van Klaveren RJ, Lammers JW, Prokop M (2007) Monitoring of smoking-induced emphysema with CT in a lung cancer screening setting: detection of real increase in extent of emphysema. Radiology 244:890–897. doi:10.1148/radiol.2443061330
Sluimer I, Prokop M, van Ginneken B (2005) Toward automated segmentation of the pathological lung in CT. IEEE Trans Med Imaging 24:1025–1038. doi:10.1109/TMI.2005.851757
Tschirren J, Hoffman EA, McLennan G, Sonka M (2005) Intrathoracic airway trees: segmentation and airway morphology analysis from low-dose CT scans. IEEE Trans Med Imaging 24:1529–1539. doi:10.1109/TMI.2005.857654
Ito S, Ingenito EP, Brewer KK, Black LD, Parameswaran H, Lutchen KR, Suki B (2005) Mechanics, nonlinearity, and failure strength of lung tissue in a mouse model of emphysema: possible role of collagen remodeling. J Appl Physiol 98:503–511. doi:10.1152/japplphysiol.00590.2004
Lutchen KR, Hantos Z, Petak F, Adamicza A, Suki B (1996) Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J Appl Physiol 80:1841–1849
Acknowledgements
This project was partially funded by the “UTE Project CIMA”, the Spanish Ministry of Health under grants PI070751, PI070792 and RTICC RD06/0020/0066, and the subprogram for unique strategic projects of the Spanish Science and Innovation Ministry (MCIIN PSS-010000-2008-2). Xabier Artaechevarria is funded by a pre-doctoral scholarship of the Basque Government. Carlos Ortiz de Solorzano and Arrate Muñoz-Barrutia hold a Ramón y Cajal Fellowship of the Spanish Ministry of Science and Innovation. We thank Prof. Joseph M. Reinhardt from the University of Iowa for his help with the automatic segmentation algorithms.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Artaechevarria, X., Blanco, D., Pérez-Martín, D. et al. Longitudinal study of a mouse model of chronic pulmonary inflammation using breath hold gated micro-CT. Eur Radiol 20, 2600–2608 (2010). https://doi.org/10.1007/s00330-010-1853-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-1853-0