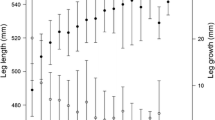
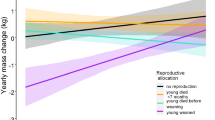
Abstract
Growth rate is a life-history trait often linked to various fitness components, including survival, age of first reproduction, and fecundity. Here we present an analysis of growth-rate variability in a wild population of savannah baboons (Papio cynocephalus). We found that relative juvenile size was a stable individual trait during the juvenile period: individuals generally remained consistently large-for-age or small-for-age throughout development. Resource availability, which varied greatly in the study population (between completely wild-foraging and partially food-enhanced social groups), had major effects on growth. Sexual maturity was accelerated for animals in the food-enhanced foraging condition, and the extent and ontogeny of sexual dimorphism differed with resource availability. Maternal characteristics also had significant effects on growth. Under both foraging conditions, females of high dominance rank and multiparous females had relatively large-for-age juveniles. Large relative juvenile size predicted earlier age of sexual maturation for both males and females in the wild-feeding condition. This confirmed that maternal effects were pervasive and contributed to differences among individuals in fitness components.




Similar content being viewed by others
References
Alberts SC, Altmann J (1995a) Balancing costs and opportunities: dispersal in male baboons. Am Nat 145:279–306
Alberts SC, Altmann J (1995b) Preparation and activation: determinants of age at reproductive maturity in male baboons. Behav Ecol Sociobiol 36:397–406
Alberts SC, Altmann J (2003) Matrix models for primate life history analysis. In: Kappeler PM, Pereira ME (eds) Primate life histories and socioecology. University of Chicago Press, Chicago, pp 66–102
Altmann J (1980) Baboon mothers and infants. Harvard University Press, Cambridge, Mass
Altmann J (1983) Costs of reproduction in baboons (Papio cynocephalus). In: Aspey WP, Lustick SI (eds) Behavioral energetics: the cost of survival in vertebrates. Ohio State University Press, Columbus, Ohio, pp 67–88
Altmann J (2000) Models of outcome and process: predicting the number of males in primate groups. In: Kappeler P (ed) Primate males. Cambridge University Press, Cambridge, pp 236–247
Altmann J, Alberts SC (1987) Body mass and growth rates in a wild primate population. Oecologia 72:15–20
Altmann J, Alberts SC (2003) Intraspecific variability in fertility and offspring survival in a non-human primate: behavioral control of ecological and social sources. In: Wachter KW, Bulatao RA (eds) Offspring: human fertility behavior in a biodemographic perspective. National Academy Press, Washington, DC, pp 140–169
Altmann J, Muruthi P (1988) Differences in daily life between semi-provisioned and wild-feeding baboons. Am J Primatol 15:213–221
Altmann J, Samuels A (1989) Upscale baboons. Nat Hist 5:60–63
Altmann J, Samuels A (1992) Costs of maternal care: infant carrying in baboons. Behav Ecol Sociobiol 29:391–398
Altmann J, Altmann SA, Hausfater G, McCuskey SA (1977) Life history of yellow baboons: physical development, reproductive parameters, and infant mortality. Primates 18:315–330
Altmann J, Altmann S, Hausfater G (1981) Physical maturation and age estimates of yellow baboons, Papio cynocephalus, in Amboseli National Park, Kenya. Am J Primatol 1:389–399
Altmann J, Altmann S, Hausfater G (1988) Determinants of reproductive success in savannah baboons (Papio cynocephalus). In: Clutton-Brock TH (ed) Reproductive success. University of Chicago Press, Chicago, pp 403–418
Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky R (1993) Body size and fatness of free-living baboons reflect food availability and activity levels. Am J Primatol 30:149–161
Altmann M, Altmann J (1991) Models of status-correlated bias in offspring sex-ratio. Am Nat 137:542–555
Altmann SA (1998) Foraging for survival. University of Chicago Press, Chicago
Arnold SJ (1992) Constraints on phenotypic evolution. Am Nat 140:S85–S107
Asquith PJ (1989) Provisioning and the study of free ranging primates—history, effects, and prospects. Yearb Phys Anthropol 32:129–158
Badyaev AV (2002) Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol Evol 17:369–378
Barker DJP (2001) Preface: type 2 diabetes: the thrifty phenotype. Br Med Bull 60:1–3
Bercovitch FB, Strum SC (1993) Dominance rank, resource availability, and reproductive maturation in female savanna baboons. Behav Ecol Sociobiol 33:313–318
Bernardo J (1996) Maternal effects in animal ecology. Am Zool 36:83–110
Bjorklund M (1990) A phylogenetic interpretation of sexual dimorphism in body size and ornament in relation to mating system in birds. J Evol Biol 3:171–183
Bloch DA, Moses LE (1988) Non-optimally weighted least squares. Am Stat 42:50–53
Bronikowski A, Altmann J (1996) Foraging in a variable environment: weather patterns and the behavioral ecology of baboons. Behav Ecol Sociobiol 39:11–25
Bulger J, Hamilton WJ III (1987) Rank and density correlates of inclusive fitness measures in a natural chacma baboon (Papio ursinus) troop. Int J Primatol 8:635–650
Case TJ (1978) On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. Q Rev Biol 53:243–282
Caswell H (2001) Matrix population models: construction, analysis and interpretation, 2nd edn. Sinauer, Sunderland, Mass
Chapais B, Berman CM (eds) (2004) Kinship and behavior in primates. Oxford University Press, Oxford
Charnov E (1991) Evolution of life history variation among female mammals. Proc Natl Acad Sci USA 88:1134–1137
Charnov EL, Berrigan D (1993) Why do female primates have such long lifespans and so few babies? or life in the slow lane. Evol Anthropol 1:191–194
Cheney DL, Seyfarth RM, Fischer J, Beehner J, Bergman T, Johnson SE, Kitchen DM, Palombit RA, Rendall D, Silk JB (2004) Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. Int J Primatol 25:401–428
Coelho AM (1985) Baboon dimorphism: growth in weight, length and adiposity from birth to 8 years of age. In: Watts ES (ed) Nonhuman primate models for human growth and development. Liss, New York, pp 125–159
Cornillon PA, Pontier D, Rochets MJ (2000) Autoregressive models for estimating phylogenetic and environmental effects: accounting for within species variation. J Theor Biol 202:247–256
Dobson FS, Oli MK (2001) The demographic basis of population regulation in Columbian ground squirrels. Am Nat 158:236–247
Dunbar RIM (1990) Environmental determinants of intraspecific variation in body weight in baboons (Papio spp.). J Zool 220:157–169
Eley RM, Strum SC, Muchemi G, Reid GDF (1989) Body condition, activity patterns, and parasitism of free-ranging troops of olive baboons (Papio anubis) in Kenya. Am J Primatol 18:209–219
Fa JE, Southwick CH (1988) Ecology and behavior of food-enhanced primate groups. Liss, New York
Festa-Bianchet M, Jorgenson JT, Reale D (2000) Early development, adult mass, and reproductive success in bighorn sheep. Behav Ecol 11:633–639
Gomendio M (1990) The influence of maternal rank and infant sex on maternal investment trends in rhesus macaques: birth sex ratios, interbirth intervals and suckling patterns. Behav Ecol Sociobiol 27:365–375
Hales CN, Barker DJP (2001) The thrifty phenotype hypothesis. Br Med Bull 60:5–20
Hamilton WJ III, Buskirk RE, Buskirk WH (1978) Omnivory and utilization of food resources by chacma baboons, Papio ursinus. Am Nat 112:911–924
Harvey PH, Purvis A (1999) Understanding the ecological and evolutionary reasons for life history variation: mammals as a case study. In: McGlade J (ed) Advanced ecological theory: principles and applications. Blackwell Science, Oxford, pp 232–247
Harvey PH, Martin RD, Clutton-Brock TH (1987) Life histories in comparative perspective. In: Smuts BB, Cheney DL, Seyfarth R, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago
Hausfater G (1975) Dominance and reproduction in baboons (Papio cynocephalus). Karger, Basel
Hedrick AV, Temeles EJ (1989) The evolution of sexual dimorphism in animals—hypotheses and tests. Trends Ecol Evol 4:136–138
Henzi SP, Lycett JE (1995) Population structure, demography, and dynamics of mountain baboons: an interim report. Am J Primatol 35:155–163
Henzi SP, Byrne RW, Whiten A (1992) Patterns of movement by baboons in the Drakensberg mountains: primary responses to the environment. Int J Primatol 13:601–629
Hofer H, East ML (1993) The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. III. Attendance and maternal care. Anim Behav 46:575–589
Janson CH, van Schaik CP (1993) Ecological risk aversion in juvenile primates: slow and steady wins the race. In: Pereira ME, Fairbanks LA (eds) Juvenile primates: life history, development, and behavior. Oxford University Press, Oxford, pp 57–74
Jarman P (1983) Mating system and sexual dimorphism in large, terrestrial, mammalian herbivores. Biol Rev 58:485–520
Johnson SE (2003) Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboons. Am J Phys Anthropol 120:83–98
Keller LF, Van Noordwijk AJ (1994) Effects of local environmental conditions on nestling growth in the great tit Parus major. Ardea 82:349–362
Kirkpatrick M, Heckman N (1989) A quantitative genetic model for growth, shape, reaction norms and other infinite-dimensional characters. J Math Biol 27:429–450
Kirkpatrick M, Lofsvold D, Bulmer M (1990) Analysis of the inheritance, selection and evolution of growth trajectories. Genetics 124:979–993
Kozlowski J, Weiner J (1997) Interspecific allometries are by-products of body size optimization. Am Nat 149:352–380
LeBlanc M, Festa-Bianchet M, Jorgenson JT (2001) Sexual size dimorphism in bighorn sheep (Ovis canadensis): effects of population density. Can J Zool 79:1661–1670
Lee PC (1996) The meanings of weaning: growth, lactation and life history. Evol Anthropol 5:87–96
Lee PC (1999) Comparative ecology of postnatal growth and weaning among haplorhine primates. In: Lee PC (ed) Comparative primate socioecology. Cambridge University Press, Cambridge, pp 111–139
Leigh SR (1992) Patterns of variation in the ontogeny of primate body size dimorphism. J Hum Evol 23:27–50
Leigh SR (1994) Ontogenetic correlates of diet in anthropoid primates. Am J Phys Anthropol 94:499–522
Leigh SR (1995) Socioecology and the ontogeny of sexual size dimorphism in anthropoid primates. Am J Phys Anthropol 97:339–356
Leigh SR, Shea BT (1996) Ontogeny of body size variation in African apes. Am J Phys Anthropol 99:43–65
Lepage D, Gauthier G, Reed A (1998) Seasonal variation in growth of greater snow goose goslings: the role of food supply. Oecologia 114:226–235
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation—a review and prospectus. Can J Zool 68:619–640
Lima SL, Zollner PA (1996) Anti-predatory vigilance and the limits to collective detection: visual and spatial separation between foragers. Behav Ecol Sociobiol 38:355–363
Lochmiller RL, Ditchkoff SS, Sinclair JA (2000) Developmental plasticity of postweanling cotton rats (Sigmodon hispidus) as an adaptation to nutritionally stochastic environments. Evol Ecol 14:127–142
Lyles AM, Dobson AP (1988) Dynamics of provisioned and unprovisioned primate populations. In: Fa JE, Southwick CH (eds) Ecology and behavior of food-enhanced primate groups. Liss, New York, pp 167–198
McAdam AG, Millar JS (1999) Dietary protein constraint on age at maturity: an experimental test with wild deer mice. J Anim Ecol 68:733–740
McNamara JM, Houston AI (1996) State-dependent life history. Nature 380:215–221
Melnick DJ, Pearl MC (1987) Cercopithecines in multimale groups: genetic diversity and population structure. In: Smuts BB, Cheney DL, Seyfarth R, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 121–134
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Mori A (1979) Analysis of population changes by measurement of body weight in the Koshima troop of Japanese monkeys. Primates 20:371–397
Moses LE, Gale LC, Altmann J (1992) Methods for analysis of unbalanced, longitudinal, growth data. Am J Primatol 28:49–59
Mosteller F, Tukey JW (1977) A method of direct assessment. In: Mosteller F, Tukey JW (eds) Data analysis and regression. Addison-Wesley, Reading, Mass, pp 133–163
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
Muruthi P (1989) Food intake and energy expenditure in savannah baboons. MSc Thesis, University of Nairobi, Kenya
Muruthi P (1997) Socioecological correlates of parental care and demography in savannah baboons. PhD Thesis, Princeton University, Princeton, NJ
Muruthi P, Altmann J, Altmann S (1991) Resource base, parity, and reproductive condition affect females’ feeding time and nutrient intake within and between groups of a baboon population. Oecologia 87:467–472
Ozanne SE, Hales CN (2004) Catch-up growth and obesity in male mice. Nature 427:411–412
Packer CP, Tatar M, Collins A (1998) Reproductive cessation in female mammals. Nature 392:807–811
Packer CP, Collins DA, Eberly LE (2000) Problems with primate sex ratios. Philos Trans R Soc Lond B Biol Sci 355:1627–1635
Pereira ME (1988) Agonistic interactions of juvenile savanna baboons. I. Fundamental features. Ethology 79:195–217
Pereira ME (1993) Juvenility in animals. In: Pereira ME, Fairbanks LA (eds) Juvenile primates: life history, development, and behavior. Oxford University Press, Oxford, pp 17–27
Pereira ME (1995) Development and social dominance among group-living primates. Am J Primatol 37:143–175
Pereira ME, Leigh SR (2003) Modes and phases of primate juvenility. In: Kappeler PM, Pereira ME (eds) Primate life histories and socioecology. University of Chicago Press, Chicago, pp 149–176
Post DG, Hausfater G, McCuskey SA (1980) Feeding behavior of yellow baboons (Papio cynocephalus): relationship to age, gender and dominance rank. Folia Primatol 34:170–195
Quinney TE, Hussell DJT, Ankney CD (1986) Sources of variation in growth of tree swallows. Auk 103:389–400
Read AF, Harvey PH (1989) Life history differences among the eutherian radiations. J Zool Lond 219:329–353
Rubenstein DI (1993) On the evolution of juvenile life-styles in mammals. In: Pereira ME, Fairbanks LA (eds) Juvenile primates: life history, development, and behavior. Oxford University Press, Oxford, pp 38–56
Samuels A, Altmann J (1984) Feeding patterns of baboon mothers (Papio cynocephalus) in Amboseli National Park, Kenya. Int J Primatol 5:376
Samuels A, Altmann J (1991) Baboons of the Amboseli basin: demographic stability and change. Int J Primatol 12:1–19
Sapolsky RM, Altmann J (1991) Incidences of hypercortisolism and dexamethasone resistance increase with age among wild baboons. Biol Psychiatr 30:1008–1016
SAS Institute (1988) SAS/STAT User’s Guide. Release 6.03 edn. SAS Institute, Cary, N.C.
Sauer JR, Slade NA (1988) Body size as a demographic categorical variable: ramifications for life history analysis of mammals. In: Boyce MS (ed) Life histories of mammals: theory and patterns. Yale University Press, New Haven, pp 107–122
Setchell JM, Lee PC, Wickings EJ, Dixson AF (2001) Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx). Am J Phys Anthropol 115:349–360
Shea BT (1990) Dynamic morphology: growth, life history, and ecology in primate evolution. In: DeRousseau CJ (ed) Primate life history and evolution. Wiley, New York, pp 325–352
Shine R (1989) Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q Rev Biol 64:419–461
Sigg H, Stolba A, Abegglen A-A, Dasser V (1982) Life history of hamadryas baboons: physical development, infant mortality, reproductive parameters and family relationships. Primates 23:473–487
Simons LS, Martin TE (1990) The hunting behaviour of individual great tits in relation to spatial variations in their food densities. Anim Behav 19:695–706
Smith JNM, Arcese P (1988) Effects of supplemental food on growth and adult size in the song sparrow. Proc XIX Int Ornithol Congr 2:1416–1423
Smuts BB, Nicolson N (1989) Reproduction in wild female olive baboons. Am J Primatol 19:229–246
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Strum SC (1991) Weight and age in wild olive baboons. Am J Primatol 25:219–237
Strum SC, Western JD (1982) Variations in fecundity with age and environment in olive baboons (Papio anubis). Am J Primatol 3:61–76
Sugiyama Y, Ohsawa H (1982) Population dynamics of Japanese monkeys with special reference to the effects of artificial feeding. Folia Primatol 39:238–263
Trivers R (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine, Chicago, pp 136–179
Walters JR, Seyfarth RM (1987) Conflict and cooperation. In: Smuts BB, Cheney DL, Seyfarth R, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 121–134
Wasser LM, Wasser SK (1995) Environmental variation and developmental rate among free ranging yellow baboons (Papio cynocephalus). Am J Primatol 35:15–30
Wasser SK, Norton GW, Kleindorfer S, Rhine RJ (2004) Population trend alters the effects of maternal dominance rank on lifetime reproductive success in yellow baboons (Papio cynocephalus). Behav Ecol Sociobiol 56:338–345
Whiten AR, Byrne RW, Henzi SP (1987) The behavioral ecology of mountain baboons. Int J Primatol 8:367–388
Whitten PL (1982) Female reproductive strategies among vervet monkeys. PhD Thesis, Harvard University
Acknowledgements
Appreciation is due to the Office of the President, Republic of Kenya, to the Kenya Wildlife Services, its Amboseli staff and Wardens, the Institute of Primate Research, and R. Leakey, D. Western, J. Else, J. Mwenda and M. Isahakia. Particular thanks go to the Amboseli fieldworkers who contributed to data collection—especially P. Muruthi, R. Mututua, A. Samuels, and S. Sayialel. Analysis was greatly facilitated by L. Moses and L. Gale and a fellowship at the Center for Advanced Study in the Behavioral Sciences with support from the John D. and Catherine T. MacArthur Foundation. Comments on an earlier draft of the manuscript were provided by S. Altmann, S. Arnold, S. Emerson, M. Festa-Bianchet, F. Janzen, S. Lenington, P. Muruthi, M. Pereira, M. Zuk, and two anonymous reviewers. For assistance with manuscript preparation, we are grateful to S.L. Combes and T. Mustra. Additional financial support was provided by NIMH15007, the National Geographic Society, the Chicago Zoological Society, and by NSF IBN-9985910 and its predecessors. This research was conducted under permit no. 13/001/C351 Vol. III from the government of Kenya and IACUC approval from the University of Chicago through 1998, and since 1998 from Princeton University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Setchell
Rights and permissions
About this article
Cite this article
Altmann, J., Alberts, S.C. Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol 57, 490–501 (2005). https://doi.org/10.1007/s00265-004-0870-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0870-x