Abstract
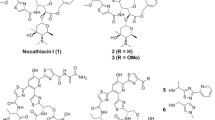
Two bacterial hosts expressing cloned aromatic oxygenases were used to catalyze the oxidation and polymerization of indole and related substrates, creating mixtures of indigoid compounds comprised of novel dimers and trimers. Crude extracts and purified compounds were tested for their ability to inhibit the growth of Gram-positive organisms, in general, and Mycobacterium tuberculosis (TB), in particular. Of the 74 compounds tested against M. tuberculosis, ~66 % had minimum inhibitory concentrations (MIC) of 5 μg/ml or less. The most effective antibiotic found was designated SAB-P1, a heterodimer of indole and anthranil, which had a MIC of 0.16 μg/ml, and did not inhibit kidney cells (IC50) at concentrations of >8 μg/ml. Combinatorial biocatalysis was used to create a series of halogenated derivatives of SAB-P1 with a wider therapeutic window. None of the derivatives had MIC values that were superior to SAB-P1, but some had a wider therapeutic window because of decreased kidney cell toxicity. Generally, the indigoid dimers that were effective against TB appeared to be specific for TB. Some of the trimers generated, however, had a broader spectrum of activity inhibiting not only TB (MIC = 1.1 μg/ml) but also the growth of Mycobacterium smegmatis MC2 155, Bacillus cereus, Enterococcus faecalis, Staphylococcus epidermidis, Bacillus subtilis 168, and Clostridium acetobutylicum. The structure of two of the novel dimers (SAB-C4 and SAB-P1) and a trimer (SAB-R1) were solved using X-ray crystallography.



Similar content being viewed by others
References
Abdel-Gawad H, Mohamed HA, Dawood KM, Badria FA (2010) Synthesis and antiviral activity of new indole-based heterocycles. Chem Pharm Bull (Tokyo) 58:1529–1531
Altreuter DH, Clark DS (1999) Combinatorial biocatalysis: taking the lead from nature. Curr Opin Biotechnol 10:130–136
Andrighetti-Fröhner CR, Antonio RV, Creczynski-Pasa TB, Barardi CR, Simõe CM (2003) Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Mem Inst Oswaldo Cruz 98:843–848
Arif SA, Poon H (2011) Tadalafil: a long-acting phosphodiesterase-5 inhibitor for the treatment of pulmonary arterial hypertension. Clin Ther 8:993–1004
Beauchard A, Ferandin Y, Frère S, Lozach O, Blairvacq M, Meijer L, Thiéry V, Besson T (2006) Synthesis of novel 5-substituted indirubins as protein kinase inhibitors. Bioorg Med Chem 18:6434–6443
Becker MH, Brucker RM, Schwantes CR, Harris RN, Minbiole KP (2009) The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl Environ Microbiol 75(21):6635–6638
Berry A, Dodge TC, Pepsin M, Weyler W (2002) Application of metabolic engineering to improve both the production and use of biotech indigo. J Ind Microbiol Biotechnol 28(3):127–133
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48(1):1–12
Capdevielle P, Maumy M (1993) 3-Oxo 3H-indole from dioxygen copper-catalyzed oxidation of indole: one-flask synthesis of 2-dalkylamino 3-oxo 3h indoles. Tetrahedron Lett 34(18):2953–2956
Chimerel C, Field CM, Piñero-Fernandez S, Keyser UF, Summers DK (2012) Indole prevents Escherichia coli cell division by modulating membrane potential. Biochim Biophys Acta 1818(7):1590–1594
Cho SH, Warit S, Wan B, Hwang CH, Pauli GF, Franzblau SG (2007) Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 51:1380–1385
Collins L, Franzblau SG (1997) Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41:1004–1009
Dalton T, Cegielski P, Akksilp S, Asencios L, Campos Caoili J, Cho SN, Erokhin VV, Ershova J, Gler MT, Kazennyy BY, Kim HJ, Kliiman K, Kurbatova E, Kvasnovsky C, Leimane V, van der Walt M, Via LE, Volchenkov GV, Yagui MA, Kang H, Global PETTS, Investigators AR, Sitti W, Wattanaamornkiet W, Andreevskaya SN, Chernousova LN, Demikhova OV, Larionova EE, Smirnova TG, Vasilieva IA, Vorobyeva AV, Barry CE 3rd, Cai Y, Shamputa IC, Bayona J, Contreras C, Bonilla C, Jave O, Brand J, Lancaster J, Odendaal R, Chen MP, Diem L, Metchock B, Tan K, Taylor A, Wolfgang M, Cho E, Eum SY, Kwak HK, Lee J, Lee J, Min S, Degtyareva I, Nemtsova ES, Khorosheva T, Kyryanova EV, Egos G, Perez MT, Tupasi T, Hwang SH, Kim CK, Kim SY, Lee HJ, Kuksa L, Norvaisha I, Skenders G, Sture I, Kummik T, Kuznetsova T, Somova T, Levina K, Pariona G, Yale G, Suarez C, Valencia E, Viiklepp P (2012) Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet 380:1406–1417
de Sá Alves FR, Barreiro EJ, Fraga CA (2003) From nature to drug discovery: the indole scaffold as a ‘privileged structure’. Mini-Rev Med Chem 7:782–793, Review
Deshpande P, Rodrigues C, Shetty A, Kapadia F, Hedge A, Soman R (2010) New Delhi Metallo-beta lactamase (NDM-1) in Enterobacteriaceae: treatment options with carbapenems compromised. J Assoc Physicians India 58:147–149
Donadio S, Maffioli S, Monciardini P, Sosio M, Jabes D (2010) Sources of novel antibiotics aside the common roads. Appl Microbiol Biotechnol 88(6):1261–1267, Review
Duarte CD, Barreiro EJ, Fraga CJ (2007) Privileged structures: a useful concept for the rational design of new lead drug candidates. Mini-Rev Med Chem 11:1108–1119
Ensley BD, Ratzkin BJ, Osslund TD, Simon MJ, Wackett LP, Gibson DT (1983) Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science 222(4620):167–169
Falzari K, Zhu Z, Pan D, Liu H, Hongmanee P, Franzblau SG (2005) In vitro and in vivo activities of macrolide derivatives against Mycobacterium tuberculosis. Antimicrob Agents Chemother 49:1447–1454
Gillespie DE, Brady SF, Bettermann AD, Cianciotto NP, Liles MR, Rondon MR, Clardy J, Goodman RM, Handelsman J (2002) Isolation of antibiotics turbomycin A and B from a metagenomic library of soil microbial DNA. Appl Environ Microbiol 68(9):4301–4306
Guengerich FP, Sorrells JL, Schmitt S, Krauser JA, Aryal P, Meijer L (2004) Generation of new protein kinase inhibitors utilizing cytochrome P450 mutant enzymes for indigoid synthesis. J Med Chem 47(12):3236–3241
Hareland WA, Crawford RL, Chapman PJ, Dagley S (1975) Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol 121(1):272–285
Harman CA, Turman MV, Kozak KR, Marnett LI, Smith WL, Garavito RM (2007) Structural basis of enantioselective inhibition of cyclooxygenase-1 by S-alpha-substituted indomethacin ethanolamides. J Biol Chem 282(38):28096–28105
Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, Niederberger W, Tang W, Eisenbrand G, Meijer L (1999) Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol 1(1):60–67
Kim JY, Kim JK, Lee SO, Kim CK, Lee K (2005) Multicomponent phenol hydroxylase-catalysed formation of hydroxyindoles and dyestuffs from indole and its derivatives. Lett Appl Microbiol 41(2):163–168
Kobayashi M, Aoki S, Gato K, Matsunami K, Kurosu M, Kitagawa I (1994) Marine natural products. XXXIV. Trisindoline, a new antibiotic indole trimer, produced by a bacterium of Vibrio sp. separated from the marine sponge Hyrtios altum. Chem. Pharm Bull (Tokyo) 42:2449–2451
Leon LL, Miranda CC, De Souza AO, Durán N (2001) Antileishmanial activity of the violacein extracted from Chromobacterium violaceum. J Antimicrob Chemother 48(3):449–450
Lopes SC, Blanco YC, Justo GZ, Nogueira PA, Rodrigues FL, Goelnitz U, Wunderlich G, Facchini G, Brocchi M, Duran N, Costa FT (2009) Violacein extracted from Chromobacterium violaceum inhibits Plasmodium growth in vitro and in vivo. Antimicrob Agents Chemother 53(5):2149–2152
Maddaford S, Renton P, Speed J, Annedi SC, Ramnauth J, Rakhit S, Andrews J, Mladenova G, Majuta L, Porreca F (2011) 1,6-Disubstituted indole derivatives as selective human neuronal nitric oxide synthase inhibitors. Bioorg Med Chem Lett 18:5234–5238
McClay K, Fox BG, Steffan RJ (1996) Chloroform mineralization by toluene-oxidizing bacteria. Appl Environ Microbiol 62:2716–2722
McClay K, Boss C, Keresztes I, Steffan RJ (2005) Mutations of toluene-4-monooxygenase that alter regiospecificity of indole oxidation and lead to production of novel indigoid pigments. Appl Environ Microbiol 71(9):5476–5483
Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P (2003) GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol 12:1255–1266
Mercadal JP, Isaac P, Siñeriz F, Ferrero MA (2010) Indigo production by Pseudomonas sp. J26, a marine naphthalene-degrading strain. J. Basic Microbiol 50(3):290–293
Mermod N, Ramos JL, Lehrbach PR, Timmis KN (1986) Vector for regulated expression of cloned genes in a wide range of gram-negative bacteria. J Bacteriol 167(2):447–454
Mojib N, Philpott R, Huang JP, Niederweis M, Bej AK (2010) Antimycobacterial activity in vitro of pigments isolated from Antarctic bacteria. Antonie Van Leeuwenhoek 98(4):531–540
Park SH, Kim DH, Jung HC, Pan JG, Ahn T, Kim D, Yun CH (2010) Engineering bacterial cytochrome P450 (P450) BM3 into a prototype with human P450 enzyme activity using indigo formation. Drug Metab Dispos 38(5):732–739
Payne DJ, Miller WH, Berry V, Brosky J, Burgess WJ, Chen E, DeWolf WE, Fosberry AP, Greenwood R, Head MS, Heerding SA, Janson CA, Jaworski DD, Keller PM, Manley PJ, Moore TD, Newlander KA, Pearson S, Polizzi BJ, Qiu X, Rittenhouse SD, Slater-Radosti C, Salyers KL, Seefeld MA, Smyth MG, Takata DT, Uzinskas IN, Vaidya K, Wallis NG, Winram SB, Yuan CC, Huffman WF (2002) Discovery of a novel and potent class of FabI-directed antibacterial agents. Antimicrob Agents Chemother 46(10):3118–3124
Pikus JD, Studts JM, Achim C, Kauffmann KE, Münck E, Steffan RJ, McClay K, Fox BG (1996) Recombinant toluene-4-monooxygenase: catalytic and Mössbauer studies of the purified diiron and rieske components of a four-protein complex. Biochemistry 35(28):9106–9119
Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio A, Roe SM, Pearl L, Leost M, Greengard P, Meijer L (2004) Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem 47(4):935–946
Priefert H, O'Brien XM, Lessard PA, Dexter AF, Choi EE, Tomic S, Nagpal G, Cho JJ, Agosto M, Yang L, Treadway SL, Tamashiro L, Wallace M, Sinskey AJ (2004) Indene bioconversion by a toluene inducible dioxygenase of Rhodococcus sp. I24. Appl. Microbiol. Biotechnology 65(2):168–176
Qu Y, Ma Q, Zhang X, Zhou H, Li X, Zhou J (2012) Optimization of indigo production by a newly isolated Pseudomonas sp. QM. J Basic Microbiol. doi:10.1002/jobm.201100516 [Epub ahead of print]
Reasoner DJ, Geldreich EE (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49(1):1–7
Reddy J, Lee C, Neeper M, Greasham R, Zhang J (1999) Development of a bioconversion process for production of cis-1S,2R-indandiol from indene by recombinant Escherichia coli constructs. Appl Microbiol Biotechnol 51:614–620
Rui L, Reardon KF, Wood TK (2005) Protein engineering of toluene ortho-monooxygenase of Burkholderia cepacia G4 for regiospecific hydroxylation of indole to form various indigoid compounds. Appl Microbiol Biotechnol 66(4):422–429
Su Y, Cheng X, Tan Y, Hu Y, Zhou Y, Liu J, Xu Y, Xie Y, Wang C, Gao Y, Wang J, Cheng T, Yang C, Xiong D, Miao H (2012) Synthesis of a dual functional anti-MDR tumor agent PH II-7 with elucidations of anti-tumor effects and mechanisms. PLoS One 7(3):E32782, Epub 2012
Takahata S, Iida M, Osaki Y, Saito J, Kitagawa H, Ozawa T, Yoshida T, Hoshiko S (2006) AG205, a novel agent directed against FabK of Streptococcus pneumoniae. Antimicrob Agents Chemother 50(8):2869–2871
Udwadia ZF (2012) Totally drug resistant tuberculosis in India: who let the djinn out? Respirology 17(5):741–742
Veluri R, Oka I, Wagner-Döbler I, Laatsch H (2003) New indole alkaloids from the North Sea bacterium Vibrio parahaemolyticus Bio249. J Nat Prod 66(11):1520–1523
Villar R, Vicente E, Solano B, Pérez-Silanes S, Aldana I, Maddry JA, Lenaerts AJ, Franzblau SG, Cho SH, Monge A, Goldman RC (2008) In vitro and in vivo antimycobacterial activities of ketone and amide derivatives of quinoxaline 1,4-di-N-oxide. J Antimicrob Chemother 62:547–554
Watsuji TO, Yamada S, Yamabe T, Watanabe Y, Kato T, Saito T, Ueda K, Beppu T (2007) Identification of indole derivatives as self-growth inhibitors of Symbiobacterium thermophilum, a unique bacterium whose growth depends on coculture with a Bacillus sp. Appl Environ Microbiol 73(19):6159–6165
Wu ZL, Aryal P, Lozach O, Meijer L, Guengerich FP (2005) Biosynthesis of new indigoid inhibitors of protein kinases using recombinant cytochrome P450 2A6. Chem Biodivers 2(1):51–65
Xu H, Wang Q, Yang WB (2010) Antifungal activities of some indole derivatives. Z Naturforsch C 65(7–8):437–439
Yoo M, Choi SU, Choi KY, Yon GH, Chae JC, Kim D, Zylstra GJ, Kim E (2008) Trisindoline synthesis and anticancer activity. Biochem Biophys Res Commun 376(1):96–99
Zoraghi R, Worrall L, See RH, Strangman W, Popplewell WL, Gong H, Samaai T, Swayze RT, Kaur S, Vuckovic M, Finlay BB, Brunham RC, McMaster WR, Davies-Coleman MT, Strynadka NC, Andersen R, Reiner NE (2011) Methicillin-resistant Staphylococcus aureus (MRSA) pyruvate kinase as a target for bis-indole alkaloids with antibacterial activities. J Biol Chem 286(52):44716–44725
Zumla A, Atun R, Maeurer M, Mwaba P, Ma Z, O'Grady J, Bates M, Dheda K, Hoelscher M, Grange J (2011) Viewpoint: Scientific dogmas, paradoxes and mysteries of latent Mycobacterium tuberculosis infection. Trop Med Int Health 16(1):79–83
Acknowledgements
This work was supported, in part, by a grant from the Global TB Alliance. We would like to thank Christopher Cooper of the Global TB Alliance for his interest and guidance, Lining Cai of Biotranex for his assistance with LC/MS, and Larry Klein of UIC for his chemistry expertise.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 448 kb)
Rights and permissions
About this article
Cite this article
McClay, K., Wan, B., Wang, Y. et al. A novel combinatorial biocatalytic approach for producing antibacterial compounds effective against Mycobacterium tuberculosis (TB). Appl Microbiol Biotechnol 97, 7151–7163 (2013). https://doi.org/10.1007/s00253-013-5012-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5012-9