Abstract
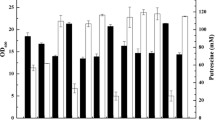
Corynebacterium glutamicum shows a great potential for the production of the polyamide monomer putrescine (1,4-diaminobutane). Previously, we constructed the putrescine-producing strain PUT1 by deletion of argF, the gene for ornithine transcarbamoylase (OTC), and argR, encoding the l-arginine repressor, combined with heterologous expression of the Escherichia coli gene for l-ornithine decarboxylase SpeC. As a consequence of argF deletion, this strain requires supplementation of l-arginine and shows growth-decoupled putrescine production. To avoid costly supplementation with l-arginine and the strong feedback inhibition of the key enzyme N-acetylglutamate kinase (ArgB) by l-arginine, a plasmid addiction system for low-level argF expression was developed. By fine-tuning argF expression through modifications of the promoter, the translational start codon and/or the ribosome binding site, high productivity and titer could be obtained. OTC activity varied almost thousandfold between 960 and 1 mU mg−1 resulting in putrescine yields on glucose from less than 0.001 up to 0.26 g g−1, the highest yield in bacteria reported to date. The most promising strain, designated PUT21, was characterized comprehensively. PUT21 strain grew with a rate of 0.19 h−1 in mineral salt medium without the need for l-arginine supplementation and produced putrescine with a yield of 0.16 g g−1 glucose at a volumetric productivity of 0.57 g L−1 h−1 and a specific productivity of 0.042 g g−1 h−1. The carbon balance suggested that no major unidentified by-product was produced. Compared to the first-generation strain PUT1, the putrescine yield observed with PUT21 was increased by 60%. In fed-batch cultivation with C. glutamicum PUT21, a putrescine titer of 19 g L−1 at a volumetric productivity of 0.55 g L−1 h−1 and a yield of 0.16 g g−1 glucose could be achieved. Moreover, while plasmid segregation of the initial strain required antibiotic selection, plasmid segregation in C. glutamicum PUT21 was fully stable for more than 60 generations without antibiotic selection even in the presence of l-arginine. The ornithine decarboxylase gene speC was expressed from this argF addiction plasmid ensuring stable putrescine production by the engineered C. glutamicum strain.




Similar content being viewed by others
References
Abe S, Takayarna K, Kinoshita S (1967) Taxonomical studies on glutamic acid producing bacteria. J Gen Appl Microbiol 13:279–301
Bethesda Research Laboratories (1986) BRL pUC host: E. coli DH5α competent cells. Focus 8:9
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Eikmanns BJ, Kleinertz E, Liebl W, Sahm H (1991) A family of Corynebacterium glutamicum/Escherichia coli shuttle vectors for cloning, controlled gene expression, and promoter probing. Gene 102:93–98
Einarsson S, Josefsson B, Lagerkvist S (1983) Determination of amino acids with 9-fluorenylmethyl chloroformate and reversed-phase liquid chromatography. J Chromatogr A 282:609–618
Friehs K (2004) Plasmid copy number and plasmid stability. Adv Biochem Eng Biotechnol 86:47–82
Georgi T, Rittmann D, Wendisch VF (2005) Lysine and glutamate production by Corynebacterium glutamicum on glucose, fructose and sucrose: roles of malic enzyme and fructose-1,6-bisphosphatase. Metab Eng 7:291–301
Glansdorff N, Xu Y (2007) Microbial arginine biosynthesis: pathway, regulation and industrial production. In: Wendisch VF (ed) Amino acid biosynthesis—pathways, regulation and metabolic engineering. Springer-Verlag, Berlin
Ikeda M, Katsumata R (1999) Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose phosphate pathway. Appl Environ Microbiol 65:2497–2502
Keilhauer C, Eggeling L, Sahm H (1993) Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J Bacteriol 175:5595–5603
Kimura E (2007) Glutamate production. In: Wendisch VF (ed) Amino acid biosynthesis—pathways, regulation and metabolic engineering. Springer-Verlag, Berlin
Kind S, Jeong WK, Schröder H, Zelder O, Wittmann C (2010) Identification and elimination of the competing N-acetyldiaminopentane pathway for improved production of diaminopentane by Corynebacterium glutamicum. Appl Environ Microbiol 76:5175–5180
Kjeldsen KR, Nielsen J (2009) In silico genome-scale reconstruction and validation of the Corynebacterium glutamicum metabolic network. Biotechnol Bioeng 102:583–597
Kroll J, Klinter S, Schneider C, Voss I, Steinbüchel A (2010) Plasmid addiction systems: perspectives and applications in biotechnology. Microb Biotechnol 3:634–657
Lee H, Yoon S, Jang H, Kim C, Kim T, Ryu W, Jung J, Park Y (2000) Effects of mixing on fed-batch fermentation of l-ornithine. J Biosci Bioeng 89:539–544
Macey R, Oster GF (2003) Berkeley Madonna 8.0. University of California at Berkeley, California
Patek M (2005) Regulation of gene expression. In: Eggeling L, Bott M (eds) Handbook of Corynebacterium glutamicum. CRC, Boca Raton
Qian Z, Xia X, Lee SY (2009) Metabolic engineering of Escherichia coli for the production of putrescine: a four carbon diamine. Biotechnol Bioeng 104:651–662
Sambrook J, Russell DW (2001) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Schneider J, Wendisch VF (2010) Putrescine production by engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 88:859–868
Schneider J, Wendisch VF (2011) Biotechnological production of polyamines by bacteria: recent achievements and future perspectives. Appl Microbiol Biotechnol 91:17–30
Schneider J, Niermann K, Wendisch VF (2011) Production of the amino acids l-glutamate, l-lysine, l-ornithine and l-arginine from arabinose by recombinant Corynebacterium glutamicum. J Biotechnol 154:191–198
Shinfuku Y, Sorpitiporn N, Sono M, Furusawa C, Hirasawa T, Shimizu H (2009) Development and experimental verification of a genome-scale metabolic model for Corynebacterium glutamicum. Microb Cell Fact 8:43
Stueber D, Bujard H (1982) Transcription from efficient promoters can interfere with plasmid replication and diminish expression of plasmid specified genes. EMBO J 1:1399–1404
van der Rest ME, Lange C, Molenaar D (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl Microbiol Biotechnol 52:541–545
Vellanoweth RL, Rabinowitz JC (1992) The influence of ribosome-binding-site elements on translational efficiency in Bacillus subtilis and Escherichia coli in vivo. Mol Microbiol 6:1105–1114
Zielenkiewicz U, Ceglowski P (2001) Mechanisms of plasmid stable maintenance with special focus on plasmid addiction systems. A Biochim Pol 48:1003–1023
Acknowledgment
We wish to acknowledge fruitful discussions with Anh Nguyen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schneider, J., Eberhardt, D. & Wendisch, V.F. Improving putrescine production by Corynebacterium glutamicum by fine-tuning ornithine transcarbamoylase activity using a plasmid addiction system. Appl Microbiol Biotechnol 95, 169–178 (2012). https://doi.org/10.1007/s00253-012-3956-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-3956-9