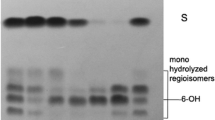
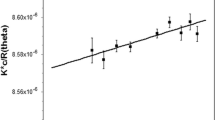
Abstract
A glucuronoyl esterase (GE) from the thermophilic fungus Sporotrichum thermophile, belonging to the carbohydrate esterase family 15 (CE-15), was functionally expressed in the methylotrophic yeast Pichia pastoris. The putative GE gene ge2 from the genomic DNA was successfully cloned in frame with the sequence for the Saccharomyces cerevisiae α-factor secretion signal under the transcriptional control of the alcohol oxidase (AOX1) promoter and integrated in P. pastoris X-33 to confirm that the encoded enzyme StGE2 exhibits esterase activity. The enzyme was active on substrates containing glucuronic acid methyl ester, showing optimal activity at pH 7.0 and 55°C. The esterase displayed broad pH range stability between 4–10 and temperature stability up to 50°C, rendering StGE2 a strong candidate for future biotechnological applications that require robust biocatalysts. ClustalW alignment of StGE2 with characterized GEs and selected homologous sequences, members of CE-15 family, revealed a novel consensus sequence G-C-S-R-X-G that features the characteristic serine residue involved in the generally conserved catalytic mechanism of the esterase family. The putative serine has been mutated, and the corresponding enzyme has been expressed in P. pastoris to prove that the candidate nucleophilic residue is responsible for catalyzing the enzymatic reaction.




Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–95
Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4:1633–49
Brenner S (1988) The molecular evolution of genes and proteins: a tale of two serines. Nature 334:528–530
Bresler MM, Rosser SJ, Basran A, Bruce NC (2000) Gene cloning and nucleotide sequencing and properties of a cocaine esterase from Rhodococcus sp. strain MB1. Appl Environ Microbiol 66:904–908
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–238
Chich J-F, Chapot-Chartier M-P, Ribadeau-Dumas B, Gripon J-C (1992) Identification of the active site serine of the X-propyl dipeptidyl aminopeptidase from Lactococcus lactis. FEBS Lett 314:139–142
Ďuranová M, Spanikova S, Wosten HAB, Biely P, de Vries RP (2009a) Two glucuronoyl esterases of Phanerochaete chrysosporium. Arch Microbiol 191:133–140
Ďuranová M, Hirsch J, Kolenová K, Biely P (2009b) Fungal glucuronoyl esterases and substrate uronic acid recognition. Biosci Biotechnol Biochem 73:2483–2487
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook. Humana Press, New Jersey, Totowa, pp 571–607
Higgins DR, Busser K, Comiskey J, Whittier PS, Purcell TJ, Hoeffler JP (1998) Small vectors for expression based on dominant drug resistance with direct multicopy selection. In: Higgins DR, Cregg JM (eds) Methods in molecular biology: Pichia protocols. Humana Press, New Jersey, Totowa, pp 28–41
Hirsch J, Kooš M, Kováč P (1998) Improved synthesis of an aldobiouronic acid related to hardwood xylans, and preparation of a derivative thereof suitable for linking to proteins. Carbohydr Res 310:145–149
Hirsch J, Langer V, Kooš M (2005) Synthesis and molecular structure of methyl 4-O-methyl-α-d-glucopyranuronate. Molecules 10:251–258
Julenius K, Mølgaard A, Gupta R, Brunak S (2005) Prediction, conservation analysis and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 15:153–64
Kabashima T, Ito K, Yoshimoto T (1996) Dipeptidyl peptidase IV from Xanthomonas maltophilia: sequencing and expression of the enzyme gene and characterization of the expressed enzyme. J Biochem 120:1111–1117
Koseki T, Miwa Y, Akao T, Akita O, Hashizume K (2006) An Aspergillus oryzae acetyl xylan esterase: molecular cloning and characteristics of recombinant enzyme expressed in Pichia pastoris. J Biotechnol 121:381–389
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) ClustalW and ClustalX version 2. Bioinformatics 23:2947–2948
Li X-L, Špániková S, de Vries RP, Biely P (2007) Identification of genes encoding microbial glucuronoyl esterases. FEBS Lett 581:4029–4035
Moukouli M, Topakas E, Christakopoulos P (2008) Cloning, characterization and functional expression of an alkalitolerant type C feruloyl esterase from Fusarium oxysporum. Appl Microbiol Biotechnol 79:245–254
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Špániková S, Biely P (2006) Glucuronoyl esterase—novel carbohydrate esterase produced by Schizophyllum commune. FEBS Lett 580:4597–4601
Stoscheck CM (1990) Quantification of protein. Meth Enzymol 182:50–68
Topakas E, Kalogeris E, Kekos D, Macris BJ, Christakopoulos P (2003) Production and partial characterization of feruloyl esterase by Sporotrichum thermophile in solid-state fermentation. Proc Biochem 38:1539–1543
Vafiadi C, Topakas E, Biely P, Christakopoulos P (2009) Purification, characterization and mass spectrometric sequencing of a thermophilic glucuronoyl esterase from Sporotrichum thermophile. FEMS Microbiol Lett 296:178–184
Vesanto E, Savijoki K, Rantanen T, Steele JL, Palva A (1995) An X-propyl dipeptidyl aminopeptidase (pepX) gene from Lactobacillus helveticus. Microbiology 141:3067–3075
Yoshpe-Besancon I, Gripon JC, Ribadeau-Dumas B (1994) Xaa-Pro dipeptidyl aminopeptidase from Lactococcus lactis catalyzes kinetically controlled synthesis of peptide bonds involving proline. Biotechnol Appl Biochem 20:131–140
Wood SJ, Li X-L, Cotta MA, Biely P, Duke NEC, Schiffer M, Pokkuluri PR (2008) Crystallization and preliminary X-ray diffraction analysis of the glucuronoyl esterase catalytic domain from Hypocrea jecorina. Acta Cryst F64:255–257
Acknowledgements
The authors thank Dr. Peter Biely from Institute of Chemistry, Slovak Academy of Sciences (Bratislava, Slovakia) who supplied the synthetic substrates for assaying GE activity.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 45 kb)
Rights and permissions
About this article
Cite this article
Topakas, E., Moukouli, M., Dimarogona, M. et al. Functional expression of a thermophilic glucuronoyl esterase from Sporotrichum thermophile: identification of the nucleophilic serine. Appl Microbiol Biotechnol 87, 1765–1772 (2010). https://doi.org/10.1007/s00253-010-2655-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2655-7