Abstract
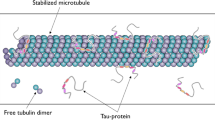
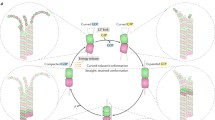
We model the dynamical states of the C-termini of tubulin dimers that comprise neuronal microtubules. We use molecular dynamics and other computational tools to explore the time-dependent behavior of conformational states of a C-terminus of tubulin within a microtubule and assume that each C-terminus interacts via screened Coulomb forces with the surface of a tubulin dimer, with neighboring C-termini and also with any adjacent microtubule-associated protein 2 (MAP2). Each C-terminus can either bind to the tubulin surface via one of the several positively charged regions or can be allowed to explore the space available in the solution surrounding the dimer. We find that the preferential orientation of each C-terminus is away from the tubulin surface but binding to the surface may also take place, albeit at a lower probability. The results of our model suggest that perturbations generated by the C-termini interactions with counterions surrounding a MAP2 may propagate over distances greater than those between adjacent microtubules. Thus, the MAP2 structure is able to act as a kind of biological wire (or a cable) transmitting local electrostatic perturbations resulting in ionic concentration gradients from one microtubule to another. We briefly discuss the implications the current dynamic modeling may have on synaptic activation and potentiation.
















Similar content being viewed by others
References
Al-Bassam J, Ozer RS, Safer D, Halpain S, Milligan RA (2002) MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J Cell Biol 157:1187–1196
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell. Garland Science Publishing, New York
Brown JA, Tuszynski JA (1999) A review of the ferroelectric model of microtubules. Ferroelectrics 220:141–155
Daune M (1999) Molecular biophysics: structures in motion. Oxford University Press, Oxford
Khuchua Z, Wozniak DF, Bardgett ME, Yue Z, McDonald M, Boero J, Hartman RE, Sims H, Strauss AW (2003) Deletion of the N-terminus of murine MAP2 by gene targeting disrupts hippocampal CA1 neuron architecture and alters contextual memory. Neuroscience 119:101–111
Kiebler MA, DesGroseillers L (2000) Molecular insights into mRNA transport and local translation in the mammalian nervous system. Neuron 25:19–28
Kim CH, Lisman JE (2001) A labile component of AMPA receptor-mediated synaptic transmission is dependent on microtubule motors, actin, and N-ethylmaleimide-sensitive factor. J Neurosci 21:4188–4194
Koradi R, Billeter M, Wuthrich K (1996) MolMol: a program for display and analysis of macromolecular structures. J Mol Graph 14:51–55, 29–32
Löwe J, Li H, Downing KH, Nogales E (2001) Refined structure of αβ-tubulin at 3.5 Å. J Mol Biol 313:1083–1095
Lu Q, Moore GD, Walss C, Luduena RF (1998) Structural and functional properties of tubulin isotypes. Adv Struct Biol 5:203–227
Makrides V, Shen TE, Bhatia R, Smith BL, Thimm J, Lal R, Feinstein SC (2003) Microtubule-dependent oligomerization of tau: implications for physiological tau function and tauopathies. J Biol Chem 278:33298–33304
Mallik B, Masunov A, Lazaridis T (2002) Distance and exposure dependent effective dielectric function. J Comp Chem 23:1090–1099
Manning G (1978) The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys 2:179–246
Nogales E, Wolf SG, Downing KH (1998) Structure of the αβ tubulin dimer by electron crystallography. Nature 391:199–203
Pedrotti B, Colombo R, Islam K (1994) Interactions of microtubule-associated protein MAP2 with unpolymerized and polymerized tubulin and actin using a 96-well microtiter plate solid-phase immunoassay. Biochemistry 33:8798–8806
Ripoll C, Norris V, Thellier M (2004) Ion condensation and signal transduction. Bioessays 26:549–557
Sackett DL (1995) Structure and function in the tubulin dimer and the role of the acid carboxyl terminus. In: Biswas BB, Roy S (eds) Subcellular biochemistry–proteins: structure, function and engineering. Kluwer, Dordrecht, 24:255–302
Steward O, Schuman EM (2001) Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci 24:299–325
Stracke R, Bohm KJ, Wollweber L, Tuszynski JA, Unger E (2002) Analysis of the migration behaviour of single microtubules in electric fields. Biochem Biophys Res Commun 293:602–609
Swope WC, Andersen HC, Berens PH, Wilson KR (1982) A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: application to small water clusters. J Chem Phys 76:637–649
Thorn KS, Ubersax JA, Vale RD (2000) Engineering the processive run length of the kinesin motor. J Cell Biol 151:1093–1100
Tokuraku K, Katsuki M, Matui T, Kuroya T, Kotani S (1999) Microtubule-binding property of microtubule-associated protein 2 differs from that of microtubule-associated protein 4 and tau. Eur J Biochem 264:996–1001
Wang Z, Sheetz MP (2000) The C-terminus of tubulin increases cytoplasmic dynein and kinesin processivity. Biophys J 78:1955–1964
Wong RW, Setou M, Teng J, Takei Y, Hirokawa N (2002) Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc Natl Acad Sci USA 99:14500–14505
Woolf NJ, Zinnerman MD, Johnson GVW (1999) Hippocampal microtubule-associated protein-2 alterations with contextual memory. Brain Res 821:241–249
Acknowledgements
This research was supported by grants from NSERC, MITACS-MMPD and the YeTaDel Foundation. We thank Mr. Eric Carpenter for assistance in computational work. Discussions with Dr. Dan Sackett of NIH are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Appendix A
Appendix A
The choice of model parameters
-
The basic units in our simulation were selected as follows:
-
Mass: 1 amu=1 D=1.66×10−27 kg
-
Time: 1 ps (1×10−12 s)
-
Charge: 1 e− (1.6×10−19 C)
-
Length: 1 nm (hence, velocity is 1 nm/ps)
-
Energy: kJ/mol
-
Force: kJ/(mol · nm)
-
-
The mass of the molecular chain of beads is estimated as 2 kD (summing up the amino acids of the C-terminus of a given human tubulin isotype), or 2000 unit masses. For simplicity we assume identical beads, i.e. for N-beads model the weight of each bead is 2 kD/N. We actually take N = 11, however the first bead is totally attached to the surface.
-
The net charge of the C-terminus is assumed to be −10e, again taken from the same isotype of tubulin.
-
The nominal length of the C-terminus is taken to be L=4.5 nm.
-
The Lennard–Jones parameters are:
-
ε=1 (however recall the truncation)
-
σ=0.45 (in nm units)
-
R c=2.01/6σ
-
-
FENE parameters:
-
K=20–30
-
R c=1.5σ
-
-
Harmonic angle force parameters:
-
K θ a =40 (the force constant)
-
θ0=135° or 3π/4
-
-
Dihedral harmonic angle force parameters:
-
K θ d =4 (the force constant)
-
ϕ0=0° (or π)
-
n=2 multiplication factor
-
-
The friction coefficient used: Γ=0.03
-
The noise was taken from a (3D) uniform distribution [−W,...,W] W=20;
Note that the friction coefficient, Γ, enters the equation of motion as part of the fluctuation-dissipation terms in the Langevin dynamics. We use the random and friction forces to represent a virtual solution without explicitly taking into account the particles in the solution. The actual parameters of the simulation are determined from the (room) temperature, the Lennard–Jones constants and the bead mass. The friction is determined as a function of the system’s time unit and the time steps of the numerical integration. In our case, we were interested in maintaining the higher vibrational frequencies, so the decay time was kept longer (determined from friction). The amplitude of the stochastic force then follows the Langevin constraints.
External force representation
The external force is simulated as a traveling localized wave with a certain amplitude, as follows: F(z(i), t)= amp sech(b(z(i)– vt))2 where z( i) is the z-position of the ith bead
Figure 8 shows the actual force on each one of the beads. The parameters of the wave were taken to be:
-
v=0.8 [nm/ps]
-
amp=6 [kJ mol−1 nm−1]
-
b=0.25
Rights and permissions
About this article
Cite this article
Priel, A., Tuszynski, J.A. & Woolf, N.J. Transitions in microtubule C-termini conformations as a possible dendritic signaling phenomenon. Eur Biophys J 35, 40–52 (2005). https://doi.org/10.1007/s00249-005-0003-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-005-0003-0