Abstract
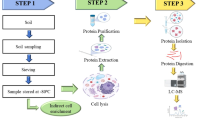
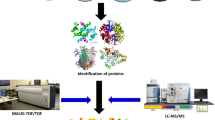
In the postgenomic era, there is a clear recognition of the limitations of nucleic acid-based methods for getting information on functions expressed by microbial communities in situ. In this context, the large-scale study of proteins expressed by indigenous microbial communities (metaproteome) should provide information to gain insights into the functioning of the microbial component in ecosystems. Characterization of the metaproteome is expected to provide data linking genetic and functional diversity of microbial communities. Studies on the metaproteome together with those on the metagenome and the metatranscriptome will contribute to progress in our knowledge of microbial communities and their contribution in ecosystem functioning. Effectiveness of the metaproteomic approach will be improved as increasing metagenomic information is made available thanks to the environmental sequencing projects currently running. More specifically, analysis of metaproteome in contrasted environmental situations should allow (1) tracking new functional genes and metabolic pathways and (2) identifying proteins preferentially associated with specific stresses. These proteins considered as functional bioindicators should contribute, in the future, to help policy makers in defining strategies for sustainable management of our environment.



Similar content being viewed by others
References
Amann, RI, Ludwig, W, Schleifer, KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. FEMS Microbiol Rev 59: 143–169
Anderson, LB, Maderia, M, Ouellette, AJA, Putman-Evans, C, Higgins, L, Krick, T, MacCoss, MJ, Lim, H, Yates, JR III, Barry, BA (2002) Post translational modifications in the CP43 subunit of photosystem II. Proc Natl Acad Sci USA 23: 14676–14681
Bakken, LR (1985) Separation and purification of bacteria from soil. Appl Environ Microbiol 49: 1482–1487
Borneman, J (1999) Culture-independent identification of microorganisms that respond to specified stimuli. Appl Environ Microbiol 65: 3398–3400
Brock, TD (1987) The study of microorganisms in situ: progress and problems. Symp Soc Gen Microbiol 41: 1–17
Cash, P, Argo, E, Ford, L, Lawrie, L, McKenzie, H (1999) A proteomic analysis of erythromycin resistance in Streptococcus pneumoniae. Electrophoresis 20: 2259–2268
Courtois, S, Frostegård, Å, Göransson, P, Depret, G, Jeannin, P, Simonet, P (2001) Quantification of bacterial subgroups in soil: comparison of DNA extracted directly from soil or from cells previously released by density gradient centrifugation. Environ Microbiol 3: 431–439
DeLong, EF (2004) Microbial population genomics and ecology: the road ahead. Environ Microbiol 6: 875–878
Ehler, MM, Cloete, TE (1999) Comparing the protein profiles of 21 different activated sludge systems after SDS-PAGE. Wat Res 33: 1181–1186
Espina, V, Woodhouse, EC, Wulkuhle, J, Asmussen, HD, Petricoin, EF III, Liotta, LA (2004) Protein microarray detection strategies: focus on direct detection technologies. J Immunol Methods 290: 121–133
Figeys, D (2000) The Achilles’ heel of proteomics. Trends Biotechnol 18: 483
Goodacre, R, Vaidyanathan, S, Dunn, WB, Harrigan, GG, Kell, DB (2004) Metabolomics by numbers: acquiring and understanding global metabolite data. Trends Biotechnol 22: 245–252
Goodlett, DR, Yi, EC (2003) Stable isotopic labeling and mass spectrometry as a means to determine differences in protein expression. Trends Anal Chem 22: 282–290
Guerreiro, N, Djordjevic, MA, Rolfe, BG (1999) Proteome analysis of the model microsymbiont Sinorhizobium meliloti: isolation and characterisation of novel proteins. Electrophoresis 20: 818–825
Gygi, SP, Corthals, GL, Zhang, Y, Rochon, Y, Aebersol, R (2000) Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology. Proc Natl Acad Sci USA 97: 9390–9395
Heim, S, Ferrer, M, Heuer, H, Regenhardt, D, Nimtz, M, Timmis, KN (2003) Proteome reference map of Pseudomonas putida strain KT2440 for genome expression profiling: distinct responses of KT2440 and Pseudomonas aeruginosa strain PAO1 to iron deprivation and a new form of superoxide dismutase. Environ Microbiol 5: 1257–1269
Hurt, RA, Qiu, X, Wu, L, Roh, Y, Palumbo, AV, Tiedje, JM, Zhou, J (2001) Simultaneous recovery of RNA and DNA from soils and sediments. Appl Environ Microbiol 67: 4495–4503
Kan, J, Hanson, TE, Ginter, JM, Wang, K, Chen, F (2005) Metaproteomic analysis of Chesapeake Bay microbial communities. Saline Systems 1: 7
Lee, KH (2001) Proteomics: a technology-driven and technology-limited discovery science. Trends Biotechnol 19: 217–222
Liesack, W, Stackebrandt, E (1992) Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol 174: 5072–5078
Manchenko, GP (1994) Handbook of Detection of Enzymes on Electroporetic Gels. CRC Press; Boca Raton, FL, pp 300
Mann, M, Pandey, A (2001) Use of mass spectrometry-derived data to annotate nucleotide and protein sequence databases. Trends Biochem Sci 26: 54–61
Maron, PA, Coeur, C, Pink, C, Clays-Josserand, A, Lensi, R, Richaume, A, Potier, P (2003) Use of polyclonal antibodies to detect and quantify the NOR protein of nitrite oxidizers in complex environments. J Microbiol Methods 53: 87–95
Maron, PA, Richaume, A, Potier, P, Lata, JC, Lensi, R (2004) Immunological method for direct assessment of the functionality of a denitrifying strain of Pseudomonas fluorescens in soil. J Microbiol Methods 58: 13–21
Maron, PA, Schimann, H, Brothier, E, Ranjard, L, Domenach, AM, Lensi, R, Nazaret, S (2006) Evaluation of quantitative and qualitative recovery of bacterial communities from different soil types by density gradient centrifugation. Eur J Soil Biol 42: 65–73
Maron, PA, Mougel, C, Siblot, S, Abbas, H, Lemanceau, P, Ranjard, L Protein extraction and fingerprinting optimization of bacterial communities in natural environment. Micob Ecol (In press)
Mounier, E, Hallet, S, Chèneby, D, Benizri, E, Gruet, Y, Nguyen, C, Piutti, S, Robin, C, Slezack-Deschaumes, S, Martin-Laurent, F, Germon, JC, Philippot, L (2004) Influence of maize mucilage on the diversity and activity of the denitrifying community. Environ Microbiol 6: 301–312
Niimi, M, Cannon, R, Monk, B (1999) Candida albicans pathogenicity: a proteomic perspective. Electrophoresis 20: 2299–2308
O’Farrell, PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250: 4007–4021
Ogunseitan, OA (1993) Direct extraction of proteins from environmental samples. J Microbiol Methods 17: 273–281
Ogunseitan, OA (1996) Protein profile in cultivated and native freshwater microorganisms exposed to chemical environmental pollutants. Microb Ecol 31: 291–304
Ogunseitan, OA (1997) Direct extraction of catalytic proteins from natural microbial communities. J Microbiol Methods 28: 55–63
Ogunseitan, OA (1998) Protein method for investigating mercuric reductase gene expression in aquatic environments. Appl Environ Microbiol 64: 695–702
Pace, NR, Stahl, DA, Olsen, GJ, Lane, DJ (1985) Analyzing natural microbial populations by rRNA sequences. Am Soc Microbiol News 51: 4–12
Pandey, A, Lewitter, F (1999) Nucleotide sequence databases: a gold mine for biologists. Trends Biochem Sci 24: 276–280
Pandey, A, Mann, M (2000) Proteomics to study genes and genomes. Nature 405: 837–846
Panicker, RC, Huang, X, Yao, SQ (2004) Recent advances in peptide-based microarray technologies. Comb Chem High Throughput Screen 7: 547–556
Pedersen, S, Bloch, PL, Reeh, S, Neidhardt, FC (1978) Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell 14: 179–190
Philippot, L (2002) Denitrifying genes in bacterial and Archeal genomes. Biochim Biophys Acta 1577: 355–376
Pimm, SL (1984) The complexity and the stability of ecosystems. Nature 307: 321–326
Radajewski, S, Ineson, P, Parekh, NR, Murrell, JC (2000) Stable-isotope probing as a tool in microbial ecology. Nature 403: 646–649
Ram, RJ, VerBerkmoes, NC, Thelen, MP, Tyson, GW, Baker, BJ, Blake, RC II, Shah, M, Hettich, RL, Banfield, JF (2005) Community proteomics of a natural microbial biofilm. Science 308: 1915–1920
Ramachandran, N, Hainsworth, E, Bhullar, B, Eisenstein, S, Rosen, B, Lau, AY, Walter, JC, LaBaer, J (2004) Self-assembling protein microarrays. Science 305: 86–90
Ranjard, L, Poly, F, Nazaret, S (2000) Monitoring complex bacterial communities using culture-independent molecular techniques: application to soil environment. Res Microbiol 151: 167–177
Rodriguez-Valera, F (2004) Environmental genomics, the big picture. FEMS Microbiol Lett 231: 153–158
Rondon, MR, August, PR, Bettermann, AD, Brady, SF, Grossman, TH, Liles, MR, Loiacono, KA, Lynch, BA, MacNeil, IA, Minor, C, Tiong, CL, Gilman, M, Osburne, MS, Clardy, J, Handelsman, J, Goodman, RM (2000) Cloning the soil metagenome: a strategy for accessing the genetic and functional diversity of uncultured microorganisms. Appl Environ Microbiol 66: 2541–2547
Schulze, WX, Gleixner, G, Kaiser, K, Guggenberger, G, Mann, M, Schulze, ED (2004) A proteomic fingerprint of dissolved organic carbon and of soil particles. Oecologia 142: 335–343
Singleton, I, Merringto, G, Colvan, S, Delahunty, JS (2003) The potential of soil protein-based methods to indicate metal contamination. Appl Soil Ecol 654: 1–8
Stein, JL, Marsh, TL, Wu, KY, Shizuya, H, DeLong, EF (1996) Characterization of uncultivated prokaryotes: isolation and analysis of a 40-kilobase-pair genome fragment from a planktonic marine archaeon. J Bacteriol 178: 591–599
Torsvik, VL, Ovreas, L (2002) Microbial diversity and function in soil: from genes to ecosystems. Curr Opin Microbiol 5: 240–245
Vasseur, C, Labadie, J, Hébraud, M (1999) Differential protein expression by Pseudomonas fragi submitted to various stresses. Electrophoresis 20: 2204–2213
Wackett, LP, Dodge, AG, Ellis, BM (2004) Microbial genomics and the periodic table. Appl Environ Microbiol 70: 647–655
Walker, BH (1992) Biodiversity and ecological redundancy. Conserv Biol 6: 18–23
Wilkins, MR, Sanchez, JC, Gooley, AA, Appel, RD, Humphery-Smith, I, Hochstrasser, DF, Williams, KL (1995) Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev 13: 19–50
Wilmes, P, Bond, PL (2004) The application of two-dimensional polyacrylamide gel electrophoresis and downstream analyses to a mixed community of prokaryotic microorganisms. Environ Microbiol 6: 911–920
Yates, JR 3rd, Speicher, S, Griffin, PR, Hunkapiller, T (1993) Peptide mass maps: a highly informative approach to protein identification. Anal Biochem 214: 397–408
Yates, JR 3rd (2004) Mass spectral analysis in proteomics. Annu Rev Biophys Biomol Struct 33: 297–316
Zhou, J, Thompson, DK (2002) Challenges in applying the microarrays to environmental studies. Curr Opin Biotechnol 13: 204–207
Acknowledgements
The authors are grateful to K. Klein for helpful comments and correcting the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maron, PA., Ranjard, L., Mougel, C. et al. Metaproteomics: A New Approach for Studying Functional Microbial Ecology. Microb Ecol 53, 486–493 (2007). https://doi.org/10.1007/s00248-006-9196-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9196-8