Abstract
Purpose
Until recently, ondansetron was approved for the prevention of nausea and vomiting only in patients older than 2 years. However, as the use of ondansetron in patients younger than 2 years had been documented, characterization of ondansetron pharmacokinetics in this younger pediatric age group was warranted.
Methods
The pharmacokinetics of intravenously administered ondansetron were evaluated in oncology and surgical patients aged 1–48 months. Pooled data from 124 patients, including 745 pharmacokinetic samples, were analyzed using nonlinear mixed-effects modeling.
Results
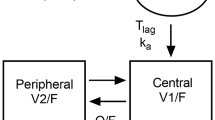
Ondansetron pharmacokinetics were described by a two-compartment model. Body-size effects on ondansetron disposition were accounted for via standard allometric relationships, normalized to 10.4 kg. A maturation process with a half-life of approximately 4 months was incorporated to describe a decrease in clearance (CL) in infants. Clearance [95% confidence interval (CI)] for a typical patient was 1.53 (1.34−1.78) L/h/kg0.75 with an interindividual variability of 56.8%. Ondansetron CL was reduced by 31%, 53%, and 76% for the typical 6-month-, 3-month-, and 1-month-old patient, respectively. Simulations showed that an ondansetron dose of 0.1 mg/kg in children younger than 6 months produced exposure similar to a 0.15-mg/kg dose in older children.
Conclusions
The population pharmacokinetic analysis of ondansetron allows for characterization of individual patients based on body weight and age. It is recommended that patients younger than 4 months receiving ondansetron be closely monitored.





Similar content being viewed by others
References
Bryson JC, Pritchard JF, Shurin S, Kernodle AE, Blumer JL (1991) Efficacy, pharmacokinetics (PK) and safety of ondansetron (OND) in pediatric chemotherapy patients (PTS). Clin Pharmacol Ther 49(2):161
Spahr-Schopfer IA, Lerman J, Sikich N, Palmer JL, Jorch U (1995) Pharmacokinetics of intravenous ondansetron in healthy children undergoing ear, nose, and throat surgery. Clin Pharmacol Ther 58(3):316–321
Pritchard J (1992) Ondansetron metabolism and pharmacokinetics. Semin Oncol 19(Suppl 10):9–15
Caron E, Bussières JF, Lebel D, Mathews S, Milot J, Jacob JL et al (2003) Ondansetron for the prevention and treatment of nausea and vomiting following pediatric strabismus surgery. Can J Ophthalmol 38(3):214–222
McQueen KD, Milton JD (1994) Multicenter postmarketing surveillance of ondansetron therapy in pediatric patients. Ann Pharmacother 28(1):85–92
Smith PV, Walton DS (2001) Prevention of vomiting after general anesthesia for pediatric ophthalmic surgery. AANA J 69(1):39–43
Khalil SN, Roth AG, Cohen IT, Simhi E, Ansermino JM, Bolos ME, Coté CJ, Hannallah RS, Davis PJ, Brooks PB, Russo MW, Anschuetz GC, Blackburn LM (2005) A double-blind comparison of intravenous ondansetron and placebo for preventing postoperative emesis in 1- to 24-month-old pediatric patients after surgery under general anesthesia. Anesth Analg 101(2):356–361
ZOFRAN Product Information (2005) GlaxoSmithKline, Research Triangle Park, NC
Finkelstein JW (1994) The effect of developmental changes in adolescence on drug disposition. J Adolesc Health 15(8):612–618
Rodman JH (1994) Pharmacokinetic variability in the adolescent: implications of body size and organ function for dosage regimen design. J Adolesc Health 15(8):654–662
Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH (2004) Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth 92(2):208–217
Hattis D, Ginsberg G, Sonawane B, Smolenski S, Russ A, Kozlak M, Goble R (2003) Differences in pharmacokinetics between children and adults–II. Children's variability in drug elimination half-lives and in some parameters needed for physiologically-based pharmacokinetic modeling. Risk Anal 23(1):117–142
Capparelli EV (1994) Pharmacokinetic considerations in the adolescent: non-cytochrome P450 metabolic pathways. J Adolesc Health 15(8):641–647
Rogers AS (1994) The role of cytochrome P450 in developmental pharmacology. J Adolesc Health 15(8):635–640
Kearns GL, Robinson PK, Wilson JT, Wilson-Costello D, Knight GR, Ward RM, van den Anker JN (2003) Cisapride disposition in neonates and infants: in vivo reflection of cytochrome P450 3A4 ontogeny. Clin Pharmacol Ther 74(4):312–325
de Wildt SN, Kearns GL, Leeder JS, van den Anker JN (1999) Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin Pharmacokinet 36(6):439–452
Bryant S, Bellamy L, Paloucek F, Wahl M (2003) Acute acetaminophen poisoning in children: kids aren't just little adults. J Emerg Med 24(4):472–473
Strolin Benedetti M, Baltes EL (2003) Drug metabolism and disposition in children. Fundam Clin Pharmacol 17(3):281–299
Linday LA (1994) Developmental changes in renal tubular function. J Adolesc Health 15(8):648–653
Linday LA, Drayer DE, Khan MA, Cicalese C, Reidenberg MM (1984) Pubertal changes in net renal tubular secretion of digoxin. Clin Pharmacol Ther 35(4):438–446
van den Anker JN (1996) Pharmacokinetics and renal function in preterm infants. Acta Paediatr 85(12):1393–1399
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med 349(12):1157–1167
Beal S, Sheiner BL (1992) NONMEM Users Guide. NONMEM Project Group, University of California at San Francisco
de Alwis DP, Aarons L, Palmer JL (1998) Population pharmacokinetics of ondansetron: a covariate analysis. Br J Clin Pharmacol 46(2):117–125
Dubois D, Du Bois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871
Anderson BJ, McKee AD, Holford NH (1997) Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin Pharmacokinet 33(5):313–327
Yano Y, Beal SL, Sheiner LB (2001) Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28(2):171–192
Ette EI (1997) Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 37(6):486–495
Ette EI, Onyiah LC (2002) Estimating inestimable standard errors in population pharmacokinetic studies: the bootstrap with Winsorization. Eur J Drug Metab Pharmacokinet 27(3):213–224
Anderson BJ, Allegaert K, Holford NH (2006) Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 165(12):819–829
Alcorn J, McNamara PJ (2002) Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin Pharmacokinet 41(12):959–998
Dixon CM, Colthup PV, Serabjit-Singh CJ, Kerr BM, Boehlert CC, Park GR et al (1995) Multiple forms of cytochrome P450 are involved in the metabolism of ondansetron in humans. Drug Metab Dispos 23(11):1225–1230
Edginton AN, Schmitt W, Voith B, Willmann S (2006) A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet 45(7):683–704
Tateishi T, Nakura H, Asoh M, Watanabe M, Tanaka M, Kumai T, Takashima S, Imaoka S, Funae Y, Yabusaki Y, Kamataki T, Kobayashi S (1997) A comparison of hepatic cytochrome P450 protein expression between infancy and postinfancy. Life Sci 61(26):2567–2574
de Wildt SN, Kearns GL, Leeder JS, van den Anker JN (1999) Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet 37(6):485–505
Sonnier M, Cresteil T (1998) Delayed ontogenesis of CYP1A2 in the human liver. Eur J Biochem 251(3):893–898
Acknowledgements
Brendan M. Johnson acknowledges support from a Clinical Pharmacokinetics/ Pharmacodynamics Fellowship provided by the University of North Carolina in collaboration with GlaxoSmithKline. The authors wish to acknowledge the following investigators of Study 1: Raafat Hannallah, Peter Davis, Joseph Tobin, and Victor Baum; and investigators who contributed pharmacokinetic data to Study 2: Jeanette Pullen, Lia Gore, Victor M. Aquino, Lisa Bomgaars, Jeffrey Blumer, Paul Gaynon, Stuart Goldman, Regina Jakacki, Mitchell Cairo, Henry Nicholson, Violet Shen, Burton Appel, James Alan Whitlock, Maxine L. Hetherington, Helen Irving, Amos Toren, Andreas Zoubek, Robert Klaassen, Purificacion Garcia-Miguel, and Arturo Munoz-Villa. The authors also wish to acknowledge Sharon C. Murray, Andrew P. Beelen, Linda M. Blackburn, Maura T. Watmuff, Vince Barnett, and Bonnie Whitehead of GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mondick, J.T., Johnson, B.M., Haberer, L.J. et al. Population pharmacokinetics of intravenous ondansetron in oncology and surgical patients aged 1−48 months. Eur J Clin Pharmacol 66, 77–86 (2010). https://doi.org/10.1007/s00228-009-0730-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0730-8