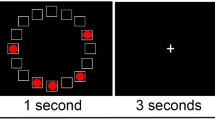
Abstract
We recently reported that visuospatial working memory capacity predicts the rate of explicit motor sequence learning (Bo and Seidler in J Neurophysiol 101:3116–3125, 2009). In the current study, we evaluated relationships between visuospatial and verbal working memory and implicit performance change in the serial reaction time (SRT) task. Participants performed two computerized working memory tasks adapted from change detection working memory assessments, an implicit SRT task, and several neuropsychological tests. We observed significant correlations between visuospatial working memory (VSWM) and verbal working memory (VWM) performance. VSWM, VWM, and card rotation task were each significantly correlated with the rate of reaction time improvement in the SRT task. Multiple linear regression analysis revealed that VSWM explained a significant portion of the variance in rate of SRT performance change (exponential fit to the performance curve) across individual participants, and the addition of VWM did not significantly improve the model. These findings suggest that VSWM plays a role in the implicit performance improvement of second-order conditional sequences.


Similar content being viewed by others
References
Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD (2010) Contributions of spatial working memory to visuomotor learning. J Cogn Neurosci 22:1917–1930
Ashe J, Lungu OV, Basford AT, Lu X (2006) Cortical control of motor sequences. Curr Opin Neurobiol 16:213–221
Awh E, Barton B, Vogel EK (2007) Visual working memory represents a fixed number of items regardless of complexity. Psychol Sci 18:622–628
Bapi RS, Miyapuram KP, Graydon FX, Doya K (2006) fMRI investigation of cortical and subcortical networks in the learning of abstract and effector-specific representations of motor sequences. Neuroimage 32:714–727
Bo J, Seidler RD (2009) Visuospatial working memory capacity predicts the organization of acquired explicit motor sequences. J Neurophysiol 101:3116–3125
Bo J, Peltier SJ, Noll DC, Seidler RD (2011) Symbolic representations in motor sequence learning. Neuroimage 54:417–426
Cohen A, Ivry RI, Keele SW (1990) Attention and structure in sequence learning. J Exp Psychol Learn Mem Cogn 16:17–30
Conway AR, Engle RW (1996) Individual differences in working memory capacity: more evidence for a general capacity theory. Memory 4:577–590
Cowan N (2001) The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci 24:87–114
Daneman M, Carpenter PA (1980) Individual-differences in working memory and reading. J Verbal Learn Verbal Behav 19:450–466
Deroost N, Soetens E (2006) The role of response selection in sequence learning. Q J Exp Psychol (Colchester) 59:449–456
Destrebecqz A, Cleeremans A (2001) Can sequence learning be implicit? New evidence with the process dissociation procedure. Psychon Bull Rev 8:343–350
Ericsson KA, Kintsch W (1995) Long-term working-memory. Psychol Rev 102:211–245
Ericsson KA, Chase WG, Faloon S (1980) Acquisition of a memory skill. Science 208:1181–1182
Frensch PA, Miner CS (1994) Effects of presentation rate and individual differences in short-term memory capacity on an indirect measure of serial learning. Mem Cogn 22:95–110
Galea JM, Albert NB, Ditye T, Miall RC (2010) Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. J Cogn Neurosci 22:1158–1164
Hazeltine E (2002) The representational nature of sequence learning: evidence for goal-based codes. In: Prinz W, Hommel B (eds) Common mechanisms in perception and action: attention and performance XIX. Oxford University Press, Oxford, pp 673–689
Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA (1993) Spatial working memory in humans as revealed by PET. Nature 363:623–625
Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS (2008) The mind and brain of short-term memory. Annu Rev Psychol 59:193–224
Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW (2004) The generality of working memory capacity: a latent-variable approach to verbal and visuospatial memory span and reasoning. J Exp Psychol Gen 133:189–217
Keele SW, Jennings P, Jones S, Caulton D, Cohen A (1995) On the modularity of sequence representation. J Mot Behav 27:17–30
Keele SW, Ivry R, Mayr U, Hazeltine E, Heuer H (2003) The cognitive and neural architecture of sequence representation. Psychol Rev 110:316–339
Kessels RP, van Zandvoort MJ, Postma A, Kappelle LJ, de Haan EH (2000) The Corsi Block-Tapping Task: standardization and normative data. Appl Neuropsychol 7:252–258
Luck SJ, Vogel EK (1997) The capacity of visual working memory for features and conjunctions. Nature 390:279–281
MacDonald MC, Christiansen MH (2002) Reassessing working memory: comment on Just and Carpenter (1992) and Waters and Caplan (1996). Psychol Rev 109:35–54
Oldfield RC (1971) Assessment and analysis of handedness—Edinburgh inventory. Neuropsychologia 9:97
Pascual-Leone A, Wassermann EM, Grafman J, Hallett M (1996) The role of the dorsolateral prefrontal cortex in implicit procedural learning. Exp Brain Res 107:479–485
Reber AS (1993) Implicit learning and tacit knowledge: an essay on the cognitive unconscious. Oxford University Press, New York
Reed J, Johnson P (1994) Assessing implicit learning with indirect tests: determining what is learned about sequence structure. J Exp Psychol Learn Mem Cogn 29:581–597
Robertson EM, Tormos JM, Maeda F, Pascual-Leone A (2001) The role of the dorsolateral prefrontal cortex during sequence learning is specific for spatial information. Cereb Cortex 11:628–635
Schumacher EH, Schwarb H (2009) Parallel response selection disrupts sequence learning under dual-task conditions. J Exp Psychol Gen 138:270–290
Schwarb H, Schumacher EH (2009) Neural evidence of a role for spatial response selection in the learning of spatial sequences. Brain Res 1247:114–125
Schwarb H, Schumacher EH (2010) Implicit sequence learning is represented by stimulus-response rules. Mem Cogn 38:677–688
Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J (2002) Cerebellum activation associated with performance change but not motor learning. Science 296:2043–2046
Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J (2005) Neural correlates of encoding and expression in implicit sequence learning. Exp Brain Res 165:114–124
Shah P, Miyake A (1996) The separability of working memory resources for spatial thinking and language processing: an individual differences approach. J Exp Psychol Gen 125:4–27
Shanks DR, Johnstone T (1999) Evaluating the relationship between explicit and implicit knowledge in a sequential reaction time task. J Exp Psychol Learn Mem Cogn 25:1435–1451
Shea CH, Park JH, Braden HW (2006) Age-related effects in sequential motor learning. Phys Ther 86:478–488
Thomason ME, Race E, Burrows B, Whitfield-Gabrieli S, Glover GH, Gabrieli JD (2009) Development of spatial and verbal working memory capacity in the human brain. J Cogn Neurosci 21:316–332
Thurston LL (1951) An analysis of mechanical aptitude. 62. University of Chicago, Psychometric Lab. Ref Type: Report
Verwey WB (1996) Buffer loading and chunking in sequential keypressing. J Exp Psychol Hum Percept Perform 22:544–562
Verwey WB (2001) Concatenating familiar movement sequences: the versatile cognitive processor. Acta Psychol (Amst) 106:69–95
Vogel EK, Machizawa MG (2004) Neural activity predicts individual differences in visual working memory capacity. Nature 428:748–751
Wechsler D (1981) Wechsler adult intelligence scale—revised. Psychological Corporation, New York
Willingham DB (1998) A neuropsychological theory of motor skill learning. Psychol Rev 105:558–584
Willingham DB, Nissen MJ, Bullemer P (1989) On the development of procedural knowledge. J Exp Psychol Learn Mem Cogn 15:1047–1060
Willingham DB, Greenberg AR, Thomas RC (1997) Response-to-stimulus interval does not affect implicit motor sequence learning, but does affect performance. Mem Cogn 25:534–542
Acknowledgments
This work was supported by National Institute of Aging grant R01 AG024106-S1 (to R. D. Seidler).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bo, J., Jennett, S. & Seidler, R.D. Working memory capacity correlates with implicit serial reaction time task performance. Exp Brain Res 214, 73–81 (2011). https://doi.org/10.1007/s00221-011-2807-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2807-8